Pretreatment with cisplatin enhances E7-specific CD8+ T-Cell-mediated antitumor immunity induced by DNA vaccination
- PMID: 18483387
- PMCID: PMC3066100
- DOI: 10.1158/1078-0432.CCR-08-0037
Pretreatment with cisplatin enhances E7-specific CD8+ T-Cell-mediated antitumor immunity induced by DNA vaccination
Abstract
Purpose: Because the combination of multiple modalities for cancer treatment is more likely to generate more potent therapeutic effects for the control of cancer, we have explored the combination of chemotherapy using cisplatin, which is routinely used in chemotherapy for advanced cervical cancer, with immunotherapy using DNA vaccines encoding calreticulin (CRT) linked to human papillomavirus type 16 E7 antigen (CRT/E7) in a preclinical model.
Experimental design: We characterized the combination of cisplatin with CRT/E7 DNA vaccine using different regimen for its potential ability to generate E7-specific CD8+ T-cell immune responses as well as antitumor effects against E7-expressing tumors.
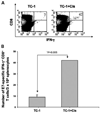
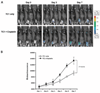
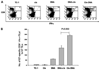
Results: Our results indicate that treatment of tumor-bearing mice with chemoimmunotherapy combining cisplatin followed by CRT/E7 DNA generated the highest E7-specific CD8+ T-cell immune response and produced the greatest antitumor effects and long-term survival as well as significant levels of E7-specific tumor-infiltrating lymphocytes compared with all the other treatment regimens. Furthermore, we found that treatment with cisplatin leads to the cell-mediated lysis of E7-expressing tumor cells in vitro and increased number of E7-specific CD8+ T-cell precursors in tumor-bearing mice. In addition, we observed that E7-specific CD8+ T cells migrate to and proliferate in the location of TC-1 tumors in mice treated with cisplatin.
Conclusions: Thus, our data suggest that chemoimmunotherapy using cisplatin followed by CRT/E7 DNA vaccine is an effective treatment against E7-expressing tumors and may potentially be translated into the clinical arena.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures




Similar articles
-
Low-dose radiation enhances therapeutic HPV DNA vaccination in tumor-bearing hosts.Cancer Immunol Immunother. 2009 May;58(5):737-48. doi: 10.1007/s00262-008-0596-0. Epub 2008 Sep 25. Cancer Immunol Immunother. 2009. PMID: 18815785 Free PMC article.
-
Histone deacetylase inhibitor AR-42 enhances E7-specific CD8⁺ T cell-mediated antitumor immunity induced by therapeutic HPV DNA vaccination.J Mol Med (Berl). 2013 Oct;91(10):1221-31. doi: 10.1007/s00109-013-1054-9. Epub 2013 May 29. J Mol Med (Berl). 2013. PMID: 23715898 Free PMC article.
-
Enhancement of vaccinia vaccine potency by linkage of tumor antigen gene to gene encoding calreticulin.Vaccine. 2004 Sep 28;22(29-30):3993-4001. doi: 10.1016/j.vaccine.2004.03.057. Vaccine. 2004. PMID: 15364449
-
Characterization of HLA-A2-restricted HPV-16 E7-specific CD8(+) T-cell immune responses induced by DNA vaccines in HLA-A2 transgenic mice.Gene Ther. 2006 Jan;13(1):67-77. doi: 10.1038/sj.gt.3302607. Gene Ther. 2006. PMID: 16107858 Free PMC article.
-
Combination regimens of radiation therapy and therapeutic cancer vaccines: mechanisms and opportunities.Semin Radiat Oncol. 2015 Jan;25(1):46-53. doi: 10.1016/j.semradonc.2014.07.002. Semin Radiat Oncol. 2015. PMID: 25481266 Free PMC article. Review.
Cited by
-
Exploitation of the propulsive force of chemotherapy for improving the response to cancer immunotherapy.Mol Oncol. 2012 Feb;6(1):1-14. doi: 10.1016/j.molonc.2011.11.005. Epub 2011 Dec 3. Mol Oncol. 2012. PMID: 22177803 Free PMC article. Review.
-
DNA vaccines for cervical cancer.Am J Transl Res. 2010 Jan 2;2(1):75-87. Am J Transl Res. 2010. PMID: 20182584 Free PMC article.
-
Combination of apigenin treatment with therapeutic HPV DNA vaccination generates enhanced therapeutic antitumor effects.J Biomed Sci. 2009 May 27;16(1):49. doi: 10.1186/1423-0127-16-49. J Biomed Sci. 2009. PMID: 19473507 Free PMC article.
-
Intralymphatic mRNA vaccine induces CD8 T-cell responses that inhibit the growth of mucosally located tumours.Sci Rep. 2016 Mar 2;6:22509. doi: 10.1038/srep22509. Sci Rep. 2016. PMID: 26931556 Free PMC article.
-
A lentiviral vector targeting a KRAS neoepitope for cancer immunotherapy.Sci Rep. 2025 Jul 2;15(1):23171. doi: 10.1038/s41598-025-05134-6. Sci Rep. 2025. PMID: 40603894 Free PMC article.
References
-
- Emens LA, Jaffee EM. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res. 2005;65:8059–8064. - PubMed
-
- Donnelly JJ, Ulmer JB, Liu MA. DNA vaccines. Life Sci. 1997;60:163–172. - PubMed
-
- Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol. 2000;18:927–974. - PubMed
-
- Hung CF, Wu TC. Improving DNA vaccine potency via modification of professional antigen presenting cells. Curr Opin Mol Ther. 2003;5:20–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

