A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells
- PMID: 18483486
- PMCID: PMC2396950
- DOI: 10.1038/embor.2008.74
A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells
Abstract
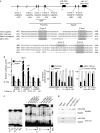
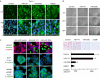
The embryonic programme 'epithelial-mesenchymal transition' (EMT) is thought to promote malignant tumour progression. The transcriptional repressor zinc-finger E-box binding homeobox 1 (ZEB1) is a crucial inducer of EMT in various human tumours, and was recently shown to promote invasion and metastasis of tumour cells. Here, we report that ZEB1 directly suppresses transcription of microRNA-200 family members miR-141 and miR-200c, which strongly activate epithelial differentiation in pancreatic, colorectal and breast cancer cells. Notably, the EMT activators transforming growth factor beta2 and ZEB1 are the predominant targets downregulated by these microRNAs. These results indicate that ZEB1 triggers an microRNA-mediated feedforward loop that stabilizes EMT and promotes invasion of cancer cells. Alternatively, depending on the environmental trigger, this loop might switch and induce epithelial differentiation, and thus explain the strong intratumorous heterogeneity observed in many human cancers.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Comment in
-
A new regulatory loop in cancer-cell invasion.EMBO Rep. 2008 Jun;9(6):521-2. doi: 10.1038/embor.2008.84. Epub 2008 May 16. EMBO Rep. 2008. PMID: 18483485 Free PMC article. No abstract available.
References
-
- Attoub S, Noe V, Pirola L, Bruyneel E, Chastre E, Mareel M, Wymann MP, Gespach C (2000) Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, rho-, and rac-dependent signaling pathways. FASEB J 14: 2329–2338 - PubMed
-
- Barrallo-Gimeno A, Nieto MA (2005) The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 132: 3151–3161 - PubMed
-
- Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297 - PubMed
-
- Bates RC, Bellovin DI, Brown C, Maynard E, Wu B, Kawakatsu H, Sheppard D, Oettgen P, Mercurio AM (2005) Transcriptional activation of integrin β6 during the epithelial–mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Invest 115: 339–347 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

