The regulation of IgA class switching
- PMID: 18483500
- PMCID: PMC3062538
- DOI: 10.1038/nri2322
The regulation of IgA class switching
Abstract
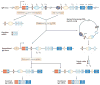
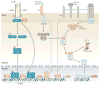
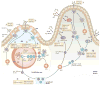
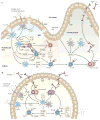
IgA class switching is the process whereby B cells acquire the expression of IgA, the most abundant antibody isotype in mucosal secretions. IgA class switching occurs via both T-cell-dependent and T-cell-independent pathways, and the antibody targets both pathogenic and commensal microorganisms. This Review describes recent advances indicating that innate immune recognition of microbial signatures at the epithelial-cell barrier is central to the selective induction of mucosal IgA class switching. In addition, the mechanisms of IgA class switching at follicular and extrafollicular sites within the mucosal environment are summarized. A better understanding of these mechanisms may help in the development of more effective mucosal vaccines.
Conflict of interest statement
The author declares no competing financial interests.
Figures

References
-
- Macpherson AJ, McKoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. An overview of the function and topography of IgA responses and the mechanisms of their induction. - PubMed
-
- Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nature Rev Immunol. 2003;3:63–72. - PubMed
-
- Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nature Rev Immunol. 2004;4:478–485. - PubMed
-
- Schlissel MS. Regulating antigen-receptor gene assembly. Nature Rev Immunol. 2003;3:890–899. - PubMed
-
- Odegard VH, Schatz DG. Targeting of somatic hypermutation. Nature Rev Immunol. 2006;6:573–583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

