Targeting of pseudorabies virus structural proteins to axons requires association of the viral Us9 protein with lipid rafts
- PMID: 18483549
- PMCID: PMC2361720
- DOI: 10.1371/journal.ppat.1000065
Targeting of pseudorabies virus structural proteins to axons requires association of the viral Us9 protein with lipid rafts
Abstract
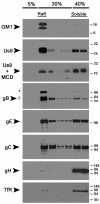
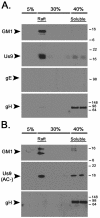
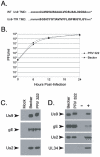
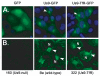
The pseudorabies virus (PRV) Us9 protein plays a central role in targeting viral capsids and glycoproteins to axons of dissociated sympathetic neurons. As a result, Us9 null mutants are defective in anterograde transmission of infection in vivo. However, it is unclear how Us9 promotes axonal sorting of so many viral proteins. It is known that the glycoproteins gB, gC, gD and gE are associated with lipid raft microdomains on the surface of infected swine kidney cells and monocytes, and are directed into the axon in a Us9-dependent manner. In this report, we determined that Us9 is associated with lipid rafts, and that this association is critical to Us9-mediated sorting of viral structural proteins. We used infected non-polarized and polarized PC12 cells, a rat pheochromocytoma cell line that acquires many of the characteristics of sympathetic neurons in the presence of nerve growth factor (NGF). In these cells, Us9 is highly enriched in detergent-resistant membranes (DRMs). Moreover, reducing the affinity of Us9 for lipid rafts inhibited anterograde transmission of infection from sympathetic neurons to epithelial cells in vitro. We conclude that association of Us9 with lipid rafts is key for efficient targeting of structural proteins to axons and, as a consequence, for directional spread of PRV from pre-synaptic to post-synaptic neurons and cells of the mammalian nervous system.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Dotti CG, Simons K. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell. 1990;62:63–72. - PubMed
-
- Edidin M. The state of lipid rafts: from model membranes to cells. Annu Rev Biophys Biomol Struct. 2003;32:257–283. - PubMed
-
- Rajendran L, Simons K. Lipid rafts and membrane dynamics. J Cell Sci. 2005;118:1099–1102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

