HOXA13 Is essential for placental vascular patterning and labyrinth endothelial specification
- PMID: 18483557
- PMCID: PMC2367452
- DOI: 10.1371/journal.pgen.1000073
HOXA13 Is essential for placental vascular patterning and labyrinth endothelial specification
Abstract
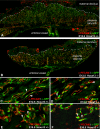
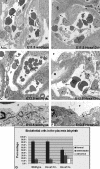
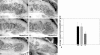
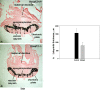
In eutherian mammals, embryonic growth and survival is dependent on the formation of the placenta, an organ that facilitates the efficient exchange of oxygen, nutrients, and metabolic waste between the maternal and fetal blood supplies. Key to the placenta's function is the formation of its vascular labyrinth, a series of finely branched vessels whose molecular ontogeny remains largely undefined. In this report, we demonstrate that HOXA13 plays an essential role in labyrinth vessel formation. In the absence of HOXA13 function, placental endothelial cell morphology is altered, causing a loss in vessel wall integrity, edema of the embryonic blood vessels, and mid-gestational lethality. Microarray analysis of wild-type and mutant placentas revealed significant changes in endothelial gene expression profiles. Notably, pro-vascular genes, including Tie2 and Foxf1, exhibited reduced expression in the mutant endothelia, which also exhibited elevated expression of genes normally expressed in lymphatic or sinusoidal endothelia. ChIP analysis of HOXA13-DNA complexes in the placenta confirmed that HOXA13 binds the Tie2 and Foxf1 promoters in vivo. In vitro, HOXA13 binds sequences present in the Tie2 and Foxf1 promoters with high affinity (K(d) = 27-42 nM) and HOXA13 can use these bound promoter regions to direct gene expression. Taken together, these findings demonstrate that HOXA13 directly regulates Tie2 and Foxf1 in the placental labyrinth endothelia, providing a functional explanation for the mid-gestational lethality exhibited by Hoxa13 mutant embryos as well as a novel transcriptional program necessary for the specification of the labyrinth vascular endothelia.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–548. - PubMed
-
- Breier G. Angiogenesis in embryonic development–a review. Placenta. 2000;21(Suppl A):S11–15. - PubMed
-
- Cross JC, Hemberger M, Lu Y, Nozaki T, Whiteley K, et al. Trophoblast functions, angiogenesis and remodeling of the maternal vasculature in the placenta. Mol Cell Endocrinol. 2002;187:207–212. - PubMed
-
- Downs KM. The murine allantois. Curr Top Dev Biol. 1998;39:1–33. - PubMed
-
- Downs KM. Early placental ontogeny in the mouse. Placenta. 2002;23:116–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

