Functional anatomy of normal bile ducts
- PMID: 18484611
- PMCID: PMC3743051
- DOI: 10.1002/ar.20664
Functional anatomy of normal bile ducts
Abstract
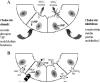
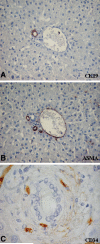
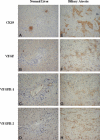
The biliary tree is a complex network of conduits that begins with the canals of Hering and progressively merges into a system of interlobular, septal, and major ducts which then coalesce to form the extrahepatic bile ducts, which finally deliver bile to the gallbladder and to the intestine. The biliary epithelium shows a morphological heterogeneity that is strictly associated with a variety of functions performed at the different levels of the biliary tree. In addition to funneling bile into the intestine, cholangiocytes (the epithelial cells lining the bile ducts) are actively involved in bile production by performing both absorbitive and secretory functions. More recently, other important biological properties restricted to cholangiocytes lining the smaller bile ducts have been outlined, with regard to their plasticity (i.e., the ability to undergo limited phenotypic changes), reactivity (i.e., the ability to participate in the inflammatory reaction to liver damage), and ability to behave as liver progenitor cells. Functional interactions with other branching systems, such as nerve and vascular structures, are crucial in the modulation of the different cholangiocyte functions.
Figures


References
-
- Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francio H, Alpini G. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132:415–431. - PubMed
-
- Benedetti A, Bassotti C, Rapino K, Marucci L, Jezequel AM. A morphometric study of the epithelium lining the rat intrahepatic biliary tree. J Hepatol. 1996;24:335–342. - PubMed
-
- Caligiuri A, Glaser S, Rodgers RE, Phinizy JL, Robertson W, Papa E, Pinzani M, Alpini G. Endothelin-1 inhibits secretinstimulated ductal secretion by interaction with ETA receptors on large cholangiocytes. Am J Physiol. 1998;275:G835–G846. - PubMed
-
- Cho WK, Boyer JL. Vasoactive intestinal polypeptide is a potent regulator of bile secretion in rat cholangiocytes. Gastroenterology. 1999a;117:420–428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

