Antibacterial colorants: characterization of prodiginines and their applications on textile materials
- PMID: 18484779
- PMCID: PMC2863012
- DOI: 10.1021/bp070481r
Antibacterial colorants: characterization of prodiginines and their applications on textile materials
Abstract
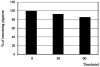
A strain of Vibrio sp. isolated from marine sediments produced large quantities of bright red pigments that could be used to dye many fibers including wool, nylon, acrylics, and silk. Characterization of the pigments by electrospray ionization mass spectrometry (ESI-MS) and nuclear magnetic resonance (NMR) revealed three prodiginine-like structures with nonpolar characteristics and low molecular mass. UV-visible spectra of the major constituent in methanol solution showed absorbance at lambda max 530 nm wavelength. The accurate mass result showed that the main isolated product has a molecular mass of m/z 323.1997. Further analysis using mass fragmentation (MS/MS), 1H NMR, COSY, HMQC NMR and DEPT confirmed the detailed structure of the pigment with an elementary composition of C20H25N3O. Fabrics dyed with the microbial prodiginines demonstrated antibacterial activity.
Figures

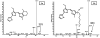

References
-
- Mapari SAS, Nielsen KF, Larsen TO, Frisvad JC, Meyer AS, Thrane U. Exploring fungal biodiversity for the production of water-soluble pigments as potential natural food colorants. Curr. Opin. Biotechnol. 2005;16(2):109–238. - PubMed
-
- Raisanen R, Nousiainen P, Hynninen PH. Dermocibin and 5-chlorodermorubin natural anthraquinone carboxylic acids as colorants for wool. Textile Res. J. 2002;72(11):973–976.
-
- Durán N, Teixeira MFS, Conti R, Esposito E. Ecological-friendly pigments from fungi. Crit. ReV. Food Sci. Nutr. 2002;42(1):53–66. De. - PubMed
-
- Hobson DK, Wales DS. Green colorants. J. Soc. Dyers Colour. 1998;114:42–44.
-
- Frandsen RJN, Nielsen NJ, Maolanon N, Sørensen JC, Olsson S, Nielsen J, Giese H. The biosynthetic pathway for aurofusarin in Fusarium graminearum reveals a close link between the naphthoquinones and naphthopyrones. Mol. Microbiol. 2006;61(4):1069–1080. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

