The statistical determinants of adaptation rate in human reaching
- PMID: 18484859
- PMCID: PMC2684526
- DOI: 10.1167/8.4.20
The statistical determinants of adaptation rate in human reaching
Abstract
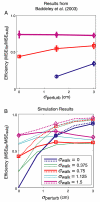
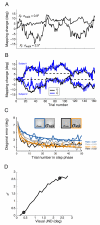
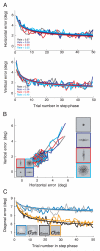
Rapid reaching to a target is generally accurate but also contains random and systematic error. Random errors result from noise in visual measurement, motor planning, and reach execution. Systematic error results from systematic changes in the mapping between the visual estimate of target location and the motor command necessary to reach the target (e.g., new spectacles, muscular fatigue). Humans maintain accurate reaching by recalibrating the visuomotor system, but no widely accepted computational model of the process exists. Given certain boundary conditions, a statistically optimal solution is a Kalman filter. We compared human to Kalman filter behavior to determine how humans take into account the statistical properties of errors and the reliability with which those errors can be measured. For most conditions, human and Kalman filter behavior was similar: Increasing measurement uncertainty caused similar decreases in recalibration rate; directionally asymmetric uncertainty caused different rates in different directions; more variation in systematic error increased recalibration rate. However, behavior differed in one respect: Inserting random error by perturbing feedback position causes slower adaptation in Kalman filters but had no effect in humans. This difference may be due to how biological systems remain responsive to changes in environmental statistics. We discuss the implications of this work.
Figures

References
-
- Adams WJ, Banks MS, van Ee R. Adaptation to three-dimensional distortions in human vision. Nature Neuroscience. 2001;4:1063–1064. [PubMed] [Article] - PubMed
-
- Alais D, Burr D. The ventriloquist effect results from near-optimal bimodal integration. Current Biology. 2004;14:257–262. [PubMed] [Article] - PubMed
-
- Bedford FL. Constraints on learning new mappings between perceptual dimensions. Journal of Experimental Psychology. 1989;15:232–248.
-
- Bedford FL. Perceptual learning. In: Medin D, editor. The psychology of learning and motivation. Academic Press; New York: 1993a. pp. 1–60.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

