Does self-administered vaginal misoprostol result in cervical ripening in postmenopausal women after 14 days of pre-treatment with estradiol? Trial protocol for a randomised, placebo-controlled sequential trial
- PMID: 18485172
- PMCID: PMC2440527
- DOI: 10.1111/j.1471-0528.2008.01727.x
Does self-administered vaginal misoprostol result in cervical ripening in postmenopausal women after 14 days of pre-treatment with estradiol? Trial protocol for a randomised, placebo-controlled sequential trial
Abstract
Objective: To compare the impact of 1000 micrograms of self-administered vaginal misoprostol versus self-administered vaginal placebo on preoperative cervical ripening after pre-treatment with estradiol vaginal tablets at home in postmenopausal women prior to day-care operative hysteroscopy.
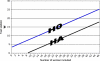
Design: Randomised double-blind placebo-controlled sequential trial. The boundaries for the sequential trial were calculated on the primary outcomes of a difference of cervical dilatation > or = 1 millimetre, with the assumption of a type 1 error of 0.05 and a power of 0.95.
Setting: Norwegian university teaching hospital.
Population: Postmenopausal women referred for day-care operative hysteroscopy.
Methods: The women were randomised to either 1000 micrograms of self-administered vaginal misoprostol or self-administered vaginal placebo the evening before day-care operative hysteroscopy. All women had administered a 25-microgram vaginal estradiol tablet daily for 14 days prior to the operation.
Main outcome measures: Preoperative cervical dilatation (difference between misoprostol and placebo group, primary outcome), difference in dilatation before and after administration of misoprostol or placebo, number of women who achieve a preoperative cervical dilatation > or = 5 millimetres, acceptability, complications and side effects (secondary outcomes).
Results: Intra-operative findings and distribution of cervical dilatation in the two treatment groups: values are given as median (range) or n (%). Difference in dilatation before and after administration of misoprostol and placebo: values are given as median (range) of intraindividual differences. Percentage of women who achieve a cervical dilatation of > or = 5 mm, percentage of women who were difficult to dilate. Acceptability in the two treatment groups: values are given as completely acceptable n (%), fairly acceptable n (%), fairly unacceptable n (%), completely unacceptable n (%). Pain in the two treatment groups: pain was measured with a visual analogue scale ranging from 0 (no pain) to 10 (unbearable pain): values are given as median (range). Occurrence of side effects in the two treatment groups. Values are given as n (%). Complications given as n (%).
Funding sources: No pharmaceutical company was involved in this study. A research grant from the regional research board of Northern Norway has been awarded to finance Dr K.S.O.'s leave from Hammerfest hospital as well as travel expenses between Hammerfest and Oslo, and research courses. The research grant from Prof B.I.N. (Helse Øst) funded the purchase of estradiol tablets, the manufacturing costs of misoprostol and placebo capsules from the hospital pharmacy, as well as the costs incurred for preparing the randomisation schedule and distribution of containers containing capsules to hospital. Prof B.I.N.'s research grant also funded insurance for the study participants.
Conclusions: Estimated completion date 31 December 2008.
Figures
Similar articles
-
A combination of misoprostol and estradiol for preoperative cervical ripening in postmenopausal women: a randomised controlled trial.BJOG. 2010 Jan;117(1):53-61. doi: 10.1111/j.1471-0528.2009.02435.x. BJOG. 2010. PMID: 20002369 Free PMC article. Clinical Trial.
-
Comparison of self-administered vaginal misoprostol versus placebo for cervical ripening prior to operative hysteroscopy using a sequential trial design.BJOG. 2008 Apr;115(5):663, e1-9. doi: 10.1111/j.1471-0528.2007.01628.x. Epub 2008 Jan 16. BJOG. 2008. PMID: 18201279 Free PMC article. Clinical Trial.
-
Comparison of self-administered vaginal misoprostol versus placebo for cervical ripening prior to operative hysteroscopy using a sequential trial design.BJOG. 2007 Jun;114(6):769, e1-12. doi: 10.1111/j.1471-0528.2007.01339.x. BJOG. 2007. PMID: 17516971 Clinical Trial.
-
Cervical ripening prior to hysteroscopy: is the application of misoprostol useful?Minerva Ginecol. 2011 Oct;63(5):439-48. Minerva Ginecol. 2011. PMID: 21926953 Review.
-
[Cervical ripening using misoprostol before hysteroscopy].Gynecol Obstet Fertil. 2006 Jan;34(1):49-53. doi: 10.1016/j.gyobfe.2005.11.004. Epub 2006 Jan 18. Gynecol Obstet Fertil. 2006. PMID: 16413811 Review. French.
Cited by
-
A combination of misoprostol and estradiol for preoperative cervical ripening in postmenopausal women: a randomised controlled trial.BJOG. 2010 Jan;117(1):53-61. doi: 10.1111/j.1471-0528.2009.02435.x. BJOG. 2010. PMID: 20002369 Free PMC article. Clinical Trial.
References
-
- Marjoribanks J, Lethaby A, Farquhar C. Surgery versus medical therapy for heavy menstrual bleeding (Cochrane review) Cochrane Database Syst Rev. 2006 CD003855. - PubMed
-
- Bradley LD. Complications in hysteroscopy: prevention, treatment and legal risk. Curr Opin Obstet Gynecol. 2002;14:409–15. - PubMed
-
- Lieng M, Qvigstad E, Sandvik L, Jørgensen H, Langebrekke A, Istre O. Hysteroscopic resection of symptomatic and asymptomatic endometrial polyps. J Minim Invasive Gynecol. 2007;14:189–94. - PubMed
-
- Grimes DA, Schulz KF, Cates WJ., Jr Prevention of uterine perforation during curettage abortion. JAMA. 1984;251:2108–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources