An adaptable hydrogel array format for 3-dimensional cell culture and analysis
- PMID: 18486205
- PMCID: PMC2490817
- DOI: 10.1016/j.biomaterials.2008.04.040
An adaptable hydrogel array format for 3-dimensional cell culture and analysis
Abstract
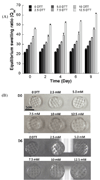
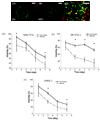
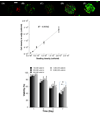
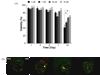
Hydrogels have been commonly used as model systems for 3-dimensional (3-D) cell biology, as they have material properties that resemble natural extracellular matrices (ECMs), and their cell-interactive properties can be readily adapted in order to address a particular hypothesis. Natural and synthetic hydrogels have been used to gain fundamental insights into virtually all aspects of cell behavior, including cell adhesion, migration, and differentiated function. However, cell responses to complex 3-D environments are difficult to adequately explore due to the large number of variables that must be controlled simultaneously. Here we describe an adaptable, automated approach for 3-D cell culture within hydrogel arrays. Our initial results demonstrate that the hydrogel network chemistry (both natural and synthetic), cell type, cell density, cell adhesion ligand density, and degradability within each array spot can be systematically varied to screen for environments that promote cell viability in a 3-D context. In a test-bed application we then demonstrate that a hydrogel array format can be used to identify environments that promote viability of HL-1 cardiomyocytes, a cell line that has not been cultured previously in 3-D hydrogel matrices. Results demonstrate that the fibronectin-derived cell adhesion ligand RGDSP improves HL-1 viability in a dose-dependent manner, and that the effect of RGDSP is particularly pronounced in degrading hydrogel arrays. Importantly, in the presence of 70mum RGDSP, HL-1 cardiomyocyte viability does not decrease even after 7 days of culture in PEG hydrogels. Taken together, our results indicate that the adaptable, array-based format developed in this study may be useful as an enhanced throughput platform for 3-D culture of a variety of cell types.
Figures





References
-
- Edelman DB, Keefer EW. A cultural renaissance: in vitro cell biology embraces three-dimensional context. Exp Neurol. 2005;192(1):1–6. - PubMed
-
- Cukierman E, Pankov R, Yamada KM. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol. 2002;14(5):633–639. - PubMed
-
- Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294(5547):1708–1712. - PubMed
-
- Vaalamo M, Mattila L, Johansson N, Kariniemi AL, Karjalainen-Lindsberg ML, Kahari VM, et al. Distinct populations of stromal cells express collagenase-3 (MMP-13) and collagenase-1 (MMP-1) in chronic ulcers but not in normally healing wounds. J Invest Dermatol. 1997;109(1):96–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

