Purinergic-mediated Ca2+ influx in Dictyostelium discoideum
- PMID: 18486207
- PMCID: PMC2658738
- DOI: 10.1016/j.ceca.2008.04.001
Purinergic-mediated Ca2+ influx in Dictyostelium discoideum
Abstract
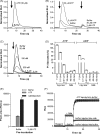
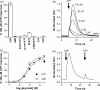
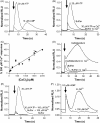
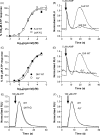
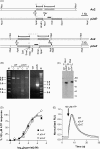
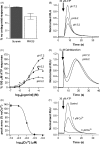
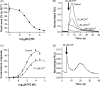
The presence of five P2X-like genes (p2xA-E) in Dictyostelium suggests that nucleotides other than cAMP may act as extracellular signalling molecules in this model eukaryote. However, p2xA was found to have an exclusively intracellular localisation making it unclear whether Dictyostelium utilise P2 receptors in a manner analogous to vertebrates. Using an apoaequorin expressing strain we show here that Dictyostelium do possess cell surface P2 receptors that facilitate Ca(2+) influx in response to extracellular ATP and ADP (EC(50)=7.5microM and 6.1microM, respectively). Indicative of P2X receptor activation, responses were rapid reaching peak within 2.91+/-0.04s, required extracellular Ca(2+), were inhibited by Gd(3+), modified by extracellular pH and were not affected by deletion of either the single Gbeta or iplA genes. Responses also remained unaffected by disruption of p2xA or p2xE showing that these genes are not involved. Cu(2+) and Zn(2+) inhibited purine-evoked Ca(2+) influx with IC(50) values of 0.9 and 6.3microM, respectively. 300microM Zn(2+) completely abolished the initial large rapid rise in intracellular Ca(2+) revealing the presence of an additional smaller, slower P2Y-like response. The existence of P2 receptors in Dictyostelium makes this organism a valuable model to explore fundamental aspects of purinergic signalling.
Figures
Similar articles
-
Functional properties of five Dictyostelium discoideum P2X receptors.J Biol Chem. 2013 Jul 19;288(29):20992-21000. doi: 10.1074/jbc.M112.445346. Epub 2013 Jun 5. J Biol Chem. 2013. PMID: 23740252 Free PMC article.
-
Functional characterization of intracellular Dictyostelium discoideum P2X receptors.J Biol Chem. 2009 Dec 11;284(50):35227-39. doi: 10.1074/jbc.M109.045674. Epub 2009 Oct 15. J Biol Chem. 2009. PMID: 19833731 Free PMC article.
-
Cross talk between P2 purinergic receptors modulates extracellular ATP-mediated interleukin-10 production in rat microglial cells.Exp Mol Med. 2008 Feb 29;40(1):19-26. doi: 10.3858/emm.2008.40.1.19. Exp Mol Med. 2008. PMID: 18305394 Free PMC article.
-
P2Y and P2X purinoceptor mediated Ca2+ signalling in glial cell pathology in the central nervous system.Eur J Pharmacol. 2002 Jul 5;447(2-3):247-60. doi: 10.1016/s0014-2999(02)01756-9. Eur J Pharmacol. 2002. PMID: 12151016 Review.
-
The P2 receptors and congenital platelet function defects.Semin Thromb Hemost. 2005 Apr;31(2):168-73. doi: 10.1055/s-2005-869522. Semin Thromb Hemost. 2005. PMID: 15852220 Review.
Cited by
-
Evidence for Extracellular ATP as a Stress Signal in a Single-Celled Organism.Eukaryot Cell. 2015 Aug;14(8):775-82. doi: 10.1128/EC.00066-15. Epub 2015 Jun 5. Eukaryot Cell. 2015. PMID: 26048010 Free PMC article.
-
A polycystin-type transient receptor potential (Trp) channel that is activated by ATP.Biol Open. 2017 Feb 15;6(2):200-209. doi: 10.1242/bio.020685. Biol Open. 2017. PMID: 28011630 Free PMC article.
-
Activation and regulation of purinergic P2X receptor channels.Pharmacol Rev. 2011 Sep;63(3):641-83. doi: 10.1124/pr.110.003129. Epub 2011 Jul 7. Pharmacol Rev. 2011. PMID: 21737531 Free PMC article. Review.
-
Proteomic analysis of extracellular ATP-regulated proteins identifies ATP synthase beta-subunit as a novel plant cell death regulator.Mol Cell Proteomics. 2011 Mar;10(3):M110.003905. doi: 10.1074/mcp.M110.003905. Epub 2010 Dec 14. Mol Cell Proteomics. 2011. PMID: 21156838 Free PMC article.
-
Extracellular signaling in Dictyostelium.Int J Dev Biol. 2019;63(8-9-10):395-405. doi: 10.1387/ijdb.190259rg. Int J Dev Biol. 2019. PMID: 31840778 Free PMC article. Review.
References
-
- Prabhu Y., Eichinger L. The Dictyostelium repertoire of seven transmembrane domain receptors. Eur. J. Cell Biol. 2006 - PubMed
-
- Taniura H., Sanada N., Kuramoto N., Yoneda Y. A metabotropic glutamate receptor family gene in Dictyostelium discoideum. J. Biol. Chem. 2006;281:12336–12343. - PubMed
-
- Anjard C., Loomis W.F. GABA induces terminal differentiation of Dictyostelium through a GABAB receptor. Development. 2006;133:2253–2261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

