Were there any "misassignments" among iron-sulfur clusters N4, N5 and N6b in NADH-quinone oxidoreductase (complex I)?
- PMID: 18486592
- PMCID: PMC2724968
- DOI: 10.1016/j.bbabio.2008.04.032
Were there any "misassignments" among iron-sulfur clusters N4, N5 and N6b in NADH-quinone oxidoreductase (complex I)?
Abstract
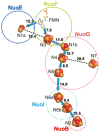
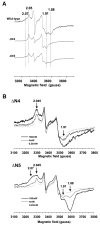
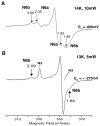
NADH-quinone oxidoreductase (complex I) in bovine heart mitochondria has a molecular weight of approximately 1 million Da composed of 45 distinct subunits. It is the largest energy transducing complex so far known. Bacterial complex I is simpler and smaller, but the essential redox components and the basic mechanisms of electron and proton translocation are the same. Over the past three decades, Ohnishi et al. have pursued extensive EPR studies near liquid helium temperatures and characterized most of the iron-sulfur clusters in complex I. Recently, Yakovlev et al. [G. Yakovlev, T. Reda, J. Hirst, Reevaluating the relationship between EPR spectra and enzyme structure for the iron-sulfur clusters in NADH:quinone oxidoreductase, Proc. Natl. Acad. Sci. U. S. A. 104 (2007) 12720-12725] challenged Ohnishi's group by claiming that there were EPR "misassignments" among clusters N4, N5 and N6b (in order to prevent confusion, we used current consensus nomenclature, as the nickname). They claimed that we misassigned EPR signals arising from cluster N5 to cluster N4, and signals from cluster N6b to cluster N4. They also proposed that cluster N5 has (4Cys)-ligands. Based on the accumulated historical data and recent results of our site-specific mutagenesis experiments, we confirmed that cluster N5 has (1His+3Cys)-ligands as we had predicted. We revealed that E. coli cluster N5 signals could be clearly detected at the sample temperature around 3 K with microwave power higher than 5 mW. Thus Hirst's group could not detect N5 signals under any of their EPR conditions, reported in their PNAS paper. It seems that they misassigned the signals from cluster N4 to N5. As to the claim of "misassignment" between clusters N4 and N6b, that was not a possibility because our mutagenesis systems did not contain cluster N6b. Therefore, we believe that we have not made any "misassignment" in our work.
Figures
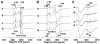
References
-
- Walker JE. The NADH:ubiquinone oxidoreductase (complex I) of respiratory chains. Q Rev Biophys. 1992;25:253–324. - PubMed
-
- Ohnishi T. Iron–sulfur clusters/semiquinones in Complex I. Biochim Biophys Acta. 1998;1364:186–206. - PubMed
-
- Yagi T, Matsuno-Yagi A. The proton-translocating NADH-quinone oxidoreductase in the respiratory chain: the secret unlocked. Biochemistry. 2003;42:2266–2274. - PubMed
-
- Sazanov LA, Hinchliffe P. Structure of the hydrophilic domain of respiratory complex I from Thernus thermophilus. Science. 2006;311:1430–1436. - PubMed
-
- Sazanov LA. Respiratory complex I: mechanistic and structural insights provided by the crystal structure of the hydrophilic domain. Biochemistry. 2007;46:2275–2288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

