Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): a multicentre randomised trial
- PMID: 18486741
- PMCID: PMC2431123
- DOI: 10.1016/S0140-6736(08)60727-8
Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): a multicentre randomised trial
Abstract
Background: Malignant pleural mesothelioma is almost always fatal, and few treatment options are available. Although active symptom control (ASC) has been recommended for the management of this disease, no consensus exists for the role of chemotherapy. We investigated whether the addition of chemotherapy to ASC improved survival and quality of life.
Methods: 409 patients with malignant pleural mesothelioma, from 76 centres in the UK and two in Australia, were randomly assigned to ASC alone (treatment could include steroids, analgesic drugs, bronchodilators, palliative radiotherapy [n=136]); to ASC plus MVP (four cycles of mitomycin 6 mg/m2, vinblastine 6 mg/m2, and cisplatin 50 mg/m2 every 3 weeks [n=137]); or to ASC plus vinorelbine (one injection of vinorelbine 30 mg/m2 every week for 12 weeks [n=136]). Randomisation was done by minimisation, with stratification for WHO performance status, histology, and centre. Follow-up was every 3 weeks to 21 weeks after randomisation, and every 8 weeks thereafter. Because of slow accrual, the two chemotherapy groups were combined and compared with ASC alone for the primary outcome of overall survival. Analysis was by intention to treat. This study is registered, number ISRCTN54469112.
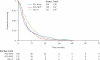
Findings: At the time of analysis, 393 (96%) patients had died (ASC 132 [97%], ASC plus MVP 132 [96%], ASC plus vinorelbine 129 [95%]). Compared with ASC alone, we noted a small, non-significant survival benefit for ASC plus chemotherapy (hazard ratio [HR] 0.89 [95% CI 0.72-1.10]; p=0.29). Median survival was 7.6 months in the ASC alone group and 8.5 months in the ASC plus chemotherapy group. Exploratory analyses suggested a survival advantage for ASC plus vinorelbine compared with ASC alone (HR 0.80 [0.63-1.02]; p=0.08), with a median survival of 9.5 months. There was no evidence of a survival benefit with ASC plus MVP (HR 0.99 [0.78-1.27]; p=0.95). We observed no between-group differences in four predefined quality-of-life subscales (physical functioning, pain, dyspnoea, and global health status) at any of the assessments in the first 6 months.
Interpretation: The addition of chemotherapy to ASC offers no significant benefits in terms of overall survival or quality of life. However, exploratory analyses suggested that vinorelbine merits further investigation.
Figures




Comment in
-
Chemotherapy for malignant pleural mesothelioma.Lancet. 2008 May 17;371(9625):1640-2. doi: 10.1016/S0140-6736(08)60703-5. Lancet. 2008. PMID: 18486725 No abstract available.
-
MVP and vinorelbine for malignant pleural mesothelioma.Lancet. 2008 Aug 23;372(9639):629; author reply 629-30. doi: 10.1016/S0140-6736(08)61273-8. Lancet. 2008. PMID: 18722865 No abstract available.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

