Head and neck cancer
- PMID: 18486742
- PMCID: PMC7720415
- DOI: 10.1016/S0140-6736(08)60728-X
Head and neck cancer
Abstract
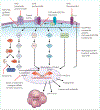
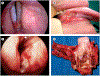
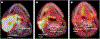
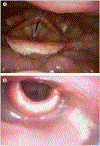
Most head and neck cancers are squamous cell carcinomas that develop in the upper aerodigestive epithelium after exposure to carcinogens such as tobacco and alcohol. Human papillomavirus has also been strongly implicated as a causative agent in a subset of these cancers. The complex anatomy and vital physiological role of the tumour-involved structures dictate that the goals of treatment are not only to improve survival outcomes but also to preserve organ function. Major improvements have been accomplished in surgical techniques and radiotherapy delivery. Moreover, systemic therapy including chemotherapy and molecularly targeted agents--namely, the epidermal growth factor receptor inhibitors--has been successfully integrated into potentially curative treatment of locally advanced squamous-cell carcinoma of the head and neck. In deciding which treatment strategy would be suitable for an individual patient, important considerations include expected functional outcomes, ability to tolerate treatment, and comorbid illnesses. The collaboration of many specialties is the key for optimum assessment and decision making. We review the epidemiology, molecular pathogenesis, diagnosis and staging, and the latest multimodal management of squamous cell carcinoma of the head and neck.
Conflict of interest statement
Conflict of interest statement
AA has been a consultant to Amgen and Imclone. He has received research support from the National Cancer Institute, Genentech, Lilly Oncology, Bristol-Myers Squibb, Pfizer, Novartis, Millenium Pharmaceuticals, M’s Science Corporation, and AstraZeneca. He also received honoraria from Genentech and AstraZeneca. MVK has no conflict of insterest to declare. DR has been a consultant to BMS, Amgen, Imclone, Lilly Oncology, Sanofi-Aventis, Array Biopharm, and GenMab. He is on the speaker’s bureau for BMS, Imclone, and Medimmune, and has received grants from AstraZeneca and Millennium Pharmaceuticals. RLF has received research support from the National Cancer Institute, the National Institute of Dental and Craniofacial Disorders, Amgen, and Altor Biosciences. He has been a consultant to Bristol-Myers Squibb, Imclone, and Altor Biosciences.
Figures

References
-
- Argiris A, Eng C. Epidemiology, staging, and screening of head and neck cancer. Cancer Treat Res 2003; 114: 15–60. - PubMed
-
- D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 2007; 356: 1944–56. - PubMed
-
- Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2004. Bethesda, MD: National Cancer Institute, 2006.
-
- Argiris A, Brockstein BE, Haraf DJ, et al. Competing causes of death and second primary tumors in patients with locoregionally advanced head and neck cancer treated with chemoradiotherapy. Clin Cancer Res 2004; 10: 1956–62. - PubMed
-
- Khuri FR, Lee JJ, Lippman SM, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst 2006; 98: 441–50. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

