Palaeoproterozoic ice houses and the evolution of oxygen-mediating enzymes: the case for a late origin of photosystem II
- PMID: 18487128
- PMCID: PMC2606766
- DOI: 10.1098/rstb.2008.0024
Palaeoproterozoic ice houses and the evolution of oxygen-mediating enzymes: the case for a late origin of photosystem II
Abstract
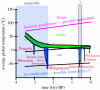
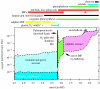
Two major geological problems regarding the origin of oxygenic photosynthesis are (i) identifying a source of oxygen pre-dating the biological oxygen production and capable of driving the evolution of oxygen tolerance, and (ii) determining when oxygenic photosynthesis evolved. One solution to the first problem is the accumulation of photochemically produced H(2)O(2) at the surface of the glaciers and its subsequent incorporation into ice. Melting at the glacier base would release H(2)O(2), which interacts with seawater to produce O(2) in an environment shielded from the lethal levels of ultraviolet radiation needed to produce H(2)O(2). Answers to the second problem are controversial and range from 3.8 to 2.2 Gyr ago. A sceptical view, based on the metals that have the redox potentials close to oxygen, argues for the late end of the range. The preponderance of geological evidence suggests little or no oxygen in the Late Archaean atmosphere (less than 1 ppm). The main piece of evidence for an earlier evolution of oxygenic photosynthesis comes from lipid biomarkers. Recent work, however, has shown that 2-methylhopanes, once thought to be unique biomarkers for cyanobacteria, are also produced anaerobically in significant quantities by at least two strains of anoxygenic phototrophs. Sterane biomarkers provide the strongest evidence for a date 2.7 Gyr ago or above, and could also be explained by the common evolutionary pattern of replacing anaerobic enzymes with oxygen-dependent ones. Although no anaerobic sterol synthesis pathway has been identified in the modern biosphere, enzymes that perform the necessary chemistry do exist. This analysis suggests that oxygenic photosynthesis could have evolved close in geological time to the Makganyene Snowball Earth Event and argues for a causal link between the two.
Figures


References
-
- Aeckersberg F, Bak F, Widdel F. Anaerobic oxidation of saturated-hydrocarbons to CO2 by a new type of sulfate-reducing bacterium. Arch. Microbiol. 1991;156:5–14. doi:10.1007/BF00418180 - DOI
-
- Aeckersberg F, Rainey F.A, Widdel F. Growth, natural relationships, cellular fatty acids and metabolic adaptation of sulfate-reducing bacteria that utilize long-chain alkanes under anoxic conditions. Arch. Microbiol. 1998;170:361–369. doi:10.1007/s002030050654 - DOI - PubMed
-
- Aitken C.M, Jones D.M, Larter S.R. Anaerobic hydrocarbon biodegradation in deep subsurface oil reservoirs. Nature. 2004;431:291–294. doi:10.1038/nature02922 - DOI - PubMed
-
- Anbar A.D, et al. A whiff of oxygen before the Great Oxidation Event? Science. 2007;317:1903–1906. doi:10.1126/science.1140325 - DOI - PubMed
-
- Andreasen A.A, Stier T.J.B. Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J. Cell. Comp. Physiol. 1953;41:23–36. doi:10.1002/jcp.1030410103 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
