Complex actions of 2-aminoethyldiphenyl borate on store-operated calcium entry
- PMID: 18487204
- PMCID: PMC2443677
- DOI: 10.1074/jbc.M801535200
Complex actions of 2-aminoethyldiphenyl borate on store-operated calcium entry
Abstract
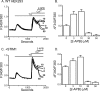
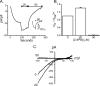
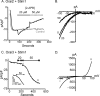
Store-operated Ca2+ entry (SOCE) is likely the most common mode of regulated influx of Ca2+ into cells. However, only a limited number of pharmacological agents have been shown to modulate this process. 2-Aminoethyldiphenyl borate (2-APB) is a widely used experimental tool that activates and then inhibits SOCE and the underlying calcium release-activated Ca2+ current (I CRAC). The mechanism by which depleted stores activates SOCE involves complex cellular movements of an endoplasmic reticulum Ca2+ sensor, STIM1, which redistributes to puncta near the plasma membrane and, in some manner, activates plasma membrane channels comprising Orai1, -2, and -3 subunits. We show here that 2-APB blocks puncta formation of fluorescently tagged STIM1 in HEK293 cells. Accordingly, 2-APB also inhibited SOCE and I(CRAC)-like currents in cells co-expressing STIM1 with the CRAC channel subunit, Orai1, with similar potency. However, 2-APB inhibited STIM1 puncta formation less well in cells co-expressing Orai1, indicating that the inhibitory effects of 2-APB are not solely dependent upon STIM1 reversal. Further, 2-APB only partially inhibited SOCE and current in cells co-expressing STIM1 and Orai2 and activated sustained currents in HEK293 cells expressing Orai3 and STIM1. Interestingly, the Orai3-dependent currents activated by 2-APB showed large outward currents at potentials greater than +50 mV. Finally, Orai3, and to a lesser extent Orai1, could be directly activated by 2-APB, independently of internal Ca2+ stores and STIM1. These data reveal novel and complex actions of 2-APB effects on SOCE that can be attributed to effects on both STIM1 as well as Orai channel subunits.
Figures






Similar articles
-
Competitive modulation of Ca2+ release-activated Ca2+ channel gating by STIM1 and 2-aminoethyldiphenyl borate.J Biol Chem. 2011 Mar 18;286(11):9429-42. doi: 10.1074/jbc.M110.189035. Epub 2010 Dec 30. J Biol Chem. 2011. PMID: 21193399 Free PMC article.
-
The ryanodine receptor agonist 4-chloro-3-ethylphenol blocks ORAI store-operated channels.Br J Pharmacol. 2014 Mar;171(5):1250-9. doi: 10.1111/bph.12528. Br J Pharmacol. 2014. PMID: 24670147 Free PMC article.
-
2-Aminoethoxydiphenyl borate directly facilitates and indirectly inhibits STIM1-dependent gating of CRAC channels.J Physiol. 2008 Jul 1;586(13):3061-73. doi: 10.1113/jphysiol.2008.151365. Epub 2008 Apr 10. J Physiol. 2008. PMID: 18403424 Free PMC article.
-
Store-Independent Orai Channels Regulated by STIM.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. PMID: 30299650 Free Books & Documents. Review.
-
Pharmacology of Store-Operated Calcium Entry Channels.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 16. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 16. PMID: 30299647 Free Books & Documents. Review.
Cited by
-
Complex actions of ionomycin in cultured cerebellar astrocytes affecting both calcium-induced calcium release and store-operated calcium entry.Neurochem Res. 2013 Jun;38(6):1260-5. doi: 10.1007/s11064-013-1021-4. Epub 2013 Mar 22. Neurochem Res. 2013. PMID: 23519933
-
Store-operated CRAC channels: function in health and disease.Nat Rev Drug Discov. 2010 May;9(5):399-410. doi: 10.1038/nrd3136. Epub 2010 Apr 16. Nat Rev Drug Discov. 2010. PMID: 20395953 Review.
-
2-APB and CBD-Mediated Targeting of Charged Cytotoxic Compounds Into Tumor Cells Suggests the Involvement of TRPV2 Channels.Front Pharmacol. 2019 Oct 15;10:1198. doi: 10.3389/fphar.2019.01198. eCollection 2019. Front Pharmacol. 2019. PMID: 31680972 Free PMC article.
-
CRAC channel gating and its modulation by STIM1 and 2-aminoethoxydiphenyl borate.J Physiol. 2017 May 15;595(10):3085-3095. doi: 10.1113/JP273130. Epub 2016 Nov 13. J Physiol. 2017. PMID: 27753099 Free PMC article.
-
Development of Store-Operated Calcium Entry-Targeted Compounds in Cancer.Front Pharmacol. 2021 May 28;12:688244. doi: 10.3389/fphar.2021.688244. eCollection 2021. Front Pharmacol. 2021. PMID: 34122115 Free PMC article. Review.
References
-
- Putney, J. W. (1986) Cell Calcium 7 1–12 - PubMed
-
- Smyth, J. T., DeHaven, W. I., Jones, B. F., Mercer, J. C., Trebak, M., Vazquez, G., and Putney, J. W. (2006) Biochim. Biophys. Acta 1763 1147–1160 - PubMed
-
- Hoth, M., and Penner, R. (1992) Nature 355 353–355 - PubMed
-
- Parekh, A. B., and Putney, J. W. (2005) Physiol. Rev. 85 757–810 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

