Transforming growth factor-beta signaling mediates hypoxia-induced pulmonary arterial remodeling and inhibition of alveolar development in newborn mouse lung
- PMID: 18487357
- PMCID: PMC2494779
- DOI: 10.1152/ajplung.00534.2007
Transforming growth factor-beta signaling mediates hypoxia-induced pulmonary arterial remodeling and inhibition of alveolar development in newborn mouse lung
Abstract
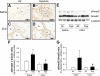
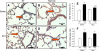
Hypoxia causes abnormal neonatal pulmonary artery remodeling (PAR) and inhibition of alveolar development (IAD). Transforming growth factor (TGF)-beta is an important regulator of lung development and repair from injury. We tested the hypothesis that inhibition of TGF-beta signaling attenuates hypoxia-induced PAR and IAD. Mice with an inducible dominant-negative mutation of the TGF-beta type II receptor (DNTGFbetaRII) and nontransgenic wild-type (WT) mice were exposed to hypoxia (12% O(2)) or air from birth to 14 days of age. Expression of DNTGFbetaRII was induced by 20 microg/g ZnSO(4) given intraperitoneally daily from birth. PAR, IAD, cell proliferation, and expression of extracellular matrix (ECM) proteins were assessed. In WT mice, hypoxia led to thicker, more muscularized resistance pulmonary arteries and impaired alveolarization, accompanied by increases in active TGF-beta and phosphorylated Smad2. Hypoxia-induced PAR and IAD were greatly attenuated in DNTGFbetaRII mice given ZnSO(4) compared with WT control mice and DNTGFbetaRII mice not given ZnSO(4). The stimulatory effects of hypoxic exposure on pulmonary arterial cell proliferation and lung ECM proteins were abrogated in DNTGFbetaRII mice given ZnSO(4). These data support the conclusion that TGF-beta plays an important role in hypoxia-induced pulmonary vascular adaptation and IAD in the newborn animal model.
Figures





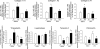

References
-
- Alejandre-Alcázar MA, Michiels-Corsten M, Vicencio AG, Reiss I, Ryu J, de Krijger RR, Haddad GG, Tibboel D, Seeger W, Eickelberg O, Morty RE. TGF-beta signaling is dynamically regulated during the alveolarization of rodent and human lungs. Dev Dyn 237: 259–269, 2008. - PubMed
-
- Alejandre-Alcázar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla-Pérez J, Wygrecka M, Eul B, Köbrich S, Hesse M, Schermuly RT, Seeger W, Eickelberg O, Morty RE. Hyperoxia modulates TGF-β/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L537–L549, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

