Peripheral refraction in normal infant rhesus monkeys
- PMID: 18487366
- PMCID: PMC2662437
- DOI: 10.1167/iovs.07-1493
Peripheral refraction in normal infant rhesus monkeys
Abstract
Purpose: To characterize peripheral refractions in infant monkeys.
Methods: Cross-sectional data for horizontal refractions were obtained from 58 normal rhesus monkeys at 3 weeks of age. Longitudinal data were obtained for both the vertical and horizontal meridians from 17 monkeys. Refractive errors were measured by retinoscopy along the pupillary axis and at eccentricities of 15 degrees , 30 degrees , and 45 degrees . Axial dimensions and corneal power were measured by ultrasonography and keratometry, respectively.
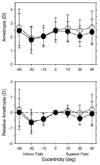
Results: In infant monkeys, the degree of radial astigmatism increased symmetrically with eccentricity in all meridians. There were, however, initial nasal-temporal and superior-inferior asymmetries in the spherical equivalent refractive errors. Specifically, the refractions in the temporal and superior fields were similar to the central ametropia, but the refractions in the nasal and inferior fields were more myopic than the central ametropia, and the relative nasal field myopia increased with the degree of central hyperopia. With age, the degree of radial astigmatism decreased in all meridians, and the refractions became more symmetrical along both the horizontal and vertical meridians. Small degrees of relative myopia were evident in all fields.
Conclusions: As in adult humans, refractive error varied as a function of eccentricity in infant monkeys and the pattern of peripheral refraction varied with the central refractive error. With age, emmetropization occurred for both central and peripheral refractive errors, resulting in similar refractions across the central 45 degrees of the visual field, which may reflect the actions of vision-dependent, growth-control mechanisms operating over a wide area of the posterior globe.
Figures







References
-
- Rempt F, Hoogerheide J, Hoogenboom WP. Peripheral retinoscopy and the skiagram. Ophthalmologica. 1971;162:1–10. - PubMed
-
- Charman WN, Jennings JA. Longitudinal changes in peripheral refraction with age. Ophthalmic Physiol Opt. 2006;26:447–455. - PubMed
-
- Lotmar W, Lotmar T. Peripheral astigmatism in the human eye: experimental data and theoretical model predictions. J Opt Soc Am. 1974;64:510–513. - PubMed
-
- Ferree CE, Rand G, Hardy C. Refraction for the peripheral field of vision. Arch Ophthalmol. 1931;5:717–731.
-
- Millodot M, Lamont A. Letter: Refraction of the periphery of the eye. J Opt Soc Am. 1974;64:110–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

