Evaluation of adenovirus-delivered human CD59 as a potential therapy for AMD in a model of human membrane attack complex formation on murine RPE
- PMID: 18487376
- PMCID: PMC4139013
- DOI: 10.1167/iovs.08-2025
Evaluation of adenovirus-delivered human CD59 as a potential therapy for AMD in a model of human membrane attack complex formation on murine RPE
Abstract
Purpose: Complement-mediated damage to the retinal pigment epithelium (RPE), Bruch membrane, and choroid has been associated with pathogenesis in age-related macular degeneration (AMD). The terminal step of complement activation involves lysis of cells by the insertion of the membrane attack complex (MAC) in the plasma membrane. The hypothesis that local overexpression of human CD59 (hCD59) delivered by an adenovirus (Ad) vector to primary murine RPE cells in vitro, RPE in vivo, or cornea ex vivo protects those cells from human MAC deposition and lysis was tested.
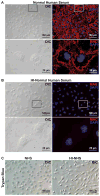
Methods: A humanized model of MAC deposition on murine cells and murine ocular tissues including RPE and cornea was developed to permit testing of human complement regulators in mice. A recombinant adenovirus-expressing hCD59 was generated, and this virus was injected into the subretinal space of adult mice. Subsequently, eyecups from these mice were exposed to human serum, and the levels of MAC deposition on the RPE were quantified. hCD59 was also expressed on murine cornea ex vivo and in murine hepatocytes, and primary RPE cells in vitro and levels of human MAC deposition and cell lysis were measured.
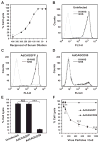
Results: Adenovirus-mediated delivery of hCD59 to the RPE, cornea, or cells in culture protects those cells from human MAC deposition and MAC-mediated damage and vesiculation.
Conclusions: The humanized model of MAC deposition on murine ocular tissues allows testing of human complement regulators that may have potential in the treatment of AMD or other diseases associated with complement activation.
Figures







Similar articles
-
A non membrane-targeted human soluble CD59 attenuates choroidal neovascularization in a model of age related macular degeneration.PLoS One. 2011 Apr 28;6(4):e19078. doi: 10.1371/journal.pone.0019078. PLoS One. 2011. PMID: 21552568 Free PMC article.
-
Decay accelerating factor (CD55)-mediated attenuation of complement: therapeutic implications for age-related macular degeneration.Invest Ophthalmol Vis Sci. 2010 Dec;51(12):6776-83. doi: 10.1167/iovs.10-5887. Epub 2010 Aug 4. Invest Ophthalmol Vis Sci. 2010. PMID: 20688727 Free PMC article.
-
Adenovirus-mediated delivery of CD46 attenuates the alternative complement pathway on RPE: implications for age-related macular degeneration.Gene Ther. 2011 Jun;18(6):613-21. doi: 10.1038/gt.2011.6. Epub 2011 Feb 10. Gene Ther. 2011. PMID: 21307887 Free PMC article.
-
The role of complement membrane attack complex in dry and wet AMD - From hypothesis to clinical trials.Exp Eye Res. 2019 Jul;184:266-277. doi: 10.1016/j.exer.2019.05.006. Epub 2019 May 10. Exp Eye Res. 2019. PMID: 31082363 Review.
-
Transplantation of the RPE in AMD.Prog Retin Eye Res. 2007 Sep;26(5):516-54. doi: 10.1016/j.preteyeres.2007.02.002. Epub 2007 Mar 6. Prog Retin Eye Res. 2007. PMID: 17532250 Review.
Cited by
-
CD59 Protects Primary Human Cerebrovascular Smooth Muscle Cells from Cytolytic Membrane Attack Complex.Brain Sci. 2024 Jun 14;14(6):601. doi: 10.3390/brainsci14060601. Brain Sci. 2024. PMID: 38928601 Free PMC article.
-
Soluble CD59 expressed from an adenovirus in vivo is a potent inhibitor of complement deposition on murine liver vascular endothelium.PLoS One. 2011;6(6):e21621. doi: 10.1371/journal.pone.0021621. Epub 2011 Jun 24. PLoS One. 2011. PMID: 21720565 Free PMC article.
-
Plasma Glycated CD59 and Gestational Diabetes Mellitus: A Systematic Review.Endocrinol Diabetes Metab. 2024 Nov;7(6):e70013. doi: 10.1002/edm2.70013. Endocrinol Diabetes Metab. 2024. PMID: 39548720 Free PMC article.
-
Enhanced retinal pigment epithelial cells as a delivery vehicle for retinal disease.Mol Ther Methods Clin Dev. 2025 Mar 14;33(2):101450. doi: 10.1016/j.omtm.2025.101450. eCollection 2025 Jun 12. Mol Ther Methods Clin Dev. 2025. PMID: 40231247 Free PMC article.
-
Drosophila GPI-mannosyltransferase 2 is required for GPI anchor attachment and surface expression of chaoptin.Vis Neurosci. 2012 May;29(3):143-56. doi: 10.1017/S0952523812000181. Epub 2012 May 10. Vis Neurosci. 2012. PMID: 22575127 Free PMC article.
References
-
- Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114:253–262. - PubMed
-
- van Leeuwen R, Klaver CC, Vingerling JR, Hofman A, de Jong PT. Epidemiology of age-related maculopathy: a review. Eur J Epidemiol. 2003;18:845–854. - PubMed
-
- Johnson LV, Ozaki S, Staples MK, Erickson PA, Anderson DH. A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res. 2000;70:441–449. - PubMed
-
- Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–732. - PubMed
-
- Johnson LV, Leitner WP, Staples MK, Anderson DH. Complement activation and inflammatory processes in drusen formation and age related macular degeneration. Exp Eye Res. 2001;73:887–896. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

