Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure
- PMID: 18487441
- PMCID: PMC2494768
- DOI: 10.1152/ajpheart.01157.2007
Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure
Abstract
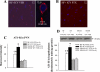
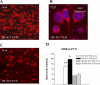
The expression of proinflammatory cytokines increases in the hypothalamus of rats with heart failure (HF). The pathophysiological significance of this observation is unknown. We hypothesized that hypothalamic proinflammatory cytokines upregulate the activity of central neural systems that contribute to increased sympathetic nerve activity in HF, specifically, the brain renin-angiotensin system (RAS) and the hypothalamic-pituitary-adrenal (HPA) axis. Rats with HF induced by coronary ligation and sham-operated controls (SHAM) were treated for 4 wk with a continuous intracerebroventricular infusion of the cytokine synthesis inhibitor pentoxifylline (PTX, 10 microg/h) or artificial cerebrospinal fluid (VEH). In VEH-treated HF rats, compared with VEH-treated SHAM rats, the hypothalamic expression of proinflammatory cytokines was increased, along with key components of the brain RAS (renin, angiotensin-converting enzyme, angiotensin type 1 receptor) and corticotropin-releasing hormone, the central indicator of HPA axis activation, in the paraventricular nucleus (PVN) of the hypothalamus. The expression of other inflammatory/excitatory mediators (superoxide, prostaglandin E(2)) was also increased, along with evidence of chronic neuronal excitation in PVN. VEH-treated HF rats had higher plasma levels of norepinephrine, ANG II, interleukin (IL)-1beta, and adrenocorticotropic hormone, increased left ventricular end-diastolic pressure, and increased wet lung-to-body weight ratio. With the exception of plasma IL-1beta, an indicator of peripheral proinflammatory cytokine activity, all measures of neurohumoral excitation were significantly lower in HF rats treated with intracerebroventricular PTX. These findings suggest that the increase in brain proinflammatory cytokines observed in rats with ischemia-induced HF is functionally significant, contributing to neurohumoral excitation by activating brain RAS and the HPA axis.
Figures





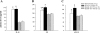
References
-
- American Physiological Society. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 283: R281–R283, 2002. - PubMed
-
- Bienvenu J, Doche C, Gutowski MC, Lenoble M, Lepape A, Perdrix JP. Production of proinflammatory cytokines and cytokines involved in the TH1/TH2 balance is modulated by pentoxifylline. J Cardiovasc Pharmacol 25, Suppl 2: S80–S84, 1995. - PubMed
-
- Bristow MR Why does the myocardium fail? Insights from basic science. Lancet 352, Suppl 1: SI8–SI14, 1998. - PubMed
-
- Brown MR, Fisher LA. Corticotropin-releasing factor: effects on the autonomic nervous system and visceral systems. Fed Proc 44: 243–248, 1985. - PubMed
-
- Catt KJ, Millan MA, Wynn PC, Mendelsohn FA, Aguilera G. Brain receptors for hypothalamic hormones. Adv Biochem Psychopharmacol 43: 51–67, 1987. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

