Reversible disruption of BCL6 repression complexes by CD40 signaling in normal and malignant B cells
- PMID: 18487509
- PMCID: PMC2481532
- DOI: 10.1182/blood-2008-01-131813
Reversible disruption of BCL6 repression complexes by CD40 signaling in normal and malignant B cells
Abstract
Germinal center (GC) B cells undergo somatic hypermutation, class switch recombination, and rapid clonal expansion to produce high-affinity antibodies. The BCL6 transcriptional repressor facilitates this phenotype because it can repress DNA damage checkpoint genes. GC B and T cells can make transient direct physical contact; T cells were observed to be associated with dead B-cell fragments. We thus hypothesized that one function of CD40 signaling from T cells within this timeframe could be to modulate BCL6 activity. CD40 signaling rapidly disrupts the ability of BCL6 to recruit the SMRT corepressor complex by excluding it from the nucleus, leading to histone acetylation, RNA polymerase II processivity, and activation of BCL6 target genes, such as CD23b, ATR, and TP53. Washout of CD40 to emulate transient T-cell contact permitted BCL6 target gene mRNA levels to return to their repressed levels, demonstrating that this is a reversible process, which could allow centroblasts that pass quality control to either continue proliferation or undergo terminal differentiation. These data suggest that transient CD40 signaling in the GC might allow T cells to weed out heavily damaged centroblasts while at the same time promoting survival of intact B cells, which could undergo differentiation or additional rounds of proliferation.
Figures
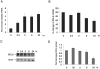
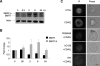
 , the slower migrating posttranslationally modified form of SMRT. Error bars represent SEM. (C) Ramos cells were transfected with a GFP-SMRT-expressing plasmid and then exposed to CD40L for 2 hours. Immunofluorescence and phase contrast microscopy were performed to determine the cellular localization of transfected SMRT. Rows 3 to 5 show experiments in which transfected Ramos cells were pretreated with the MEK kinase inhibitors PD98059 and UO126 or vehicle, followed by exposure to CD40L.
, the slower migrating posttranslationally modified form of SMRT. Error bars represent SEM. (C) Ramos cells were transfected with a GFP-SMRT-expressing plasmid and then exposed to CD40L for 2 hours. Immunofluorescence and phase contrast microscopy were performed to determine the cellular localization of transfected SMRT. Rows 3 to 5 show experiments in which transfected Ramos cells were pretreated with the MEK kinase inhibitors PD98059 and UO126 or vehicle, followed by exposure to CD40L.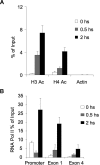
 ), and 2 hours (■). QPCR was performed to detect the abundance of CD23b promoter sequence as in Figure 2. The y-axis represents the percentage enrichment by each antibody relative to input. (B) QChIP was performed at the indicated time points using RNA POLII antibodies to detect the abundance of POLII at the CD23b promoter, exon1 and exon 4. The y-axis represents the percentage enrichment of the amplicons relative to input. The result shows increase occupancy by POLII at exon 1 and exon 4 after CD40L exposure, consistent with active transcription of the gene. Each experiment was carried out in duplicate. Error bars represent SEM.
), and 2 hours (■). QPCR was performed to detect the abundance of CD23b promoter sequence as in Figure 2. The y-axis represents the percentage enrichment by each antibody relative to input. (B) QChIP was performed at the indicated time points using RNA POLII antibodies to detect the abundance of POLII at the CD23b promoter, exon1 and exon 4. The y-axis represents the percentage enrichment of the amplicons relative to input. The result shows increase occupancy by POLII at exon 1 and exon 4 after CD40L exposure, consistent with active transcription of the gene. Each experiment was carried out in duplicate. Error bars represent SEM.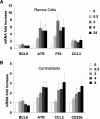
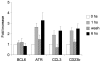
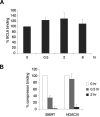
 ), the second was washed to remove the CD40L and then cultured for an additional 5 hours (▨), and the third fraction remained in CD40L containing media for another 5 hours (■). QPCR was performed in untreated cells and all 3 fractions to detect mRNA abundance of BCL6, ATR, CCL3, and CD23b. The mRNA levels of these genes were first normalized to GAPDH mRNA levels and expressed as fold increase relative to untreated cells. The experiment was performed in triplicate. Error bars represent SEM.
), the second was washed to remove the CD40L and then cultured for an additional 5 hours (▨), and the third fraction remained in CD40L containing media for another 5 hours (■). QPCR was performed in untreated cells and all 3 fractions to detect mRNA abundance of BCL6, ATR, CCL3, and CD23b. The mRNA levels of these genes were first normalized to GAPDH mRNA levels and expressed as fold increase relative to untreated cells. The experiment was performed in triplicate. Error bars represent SEM.
 ) and loss of their association with BCL6. This leads to a failure to maintain silencing of these genes, which can now become reactivated even in the presence of BCL6. The double arrow between dark zone and light zone indicates that this is a reversible mechanism. This mechanism is rapid and may allow damaged B cells to be removed from the GC reaction. (C) In the second mechanism, sustained CD40 signaling, for example, by follicular dendritic cells, can activate NFκB, which in turn induces expression of IRF4, which can then directly repress BCL6 mRNA expression leading to down-regulation of BCL6 and up-regulation of its target genes. This mechanism is slower but is irreversible and leads to differentiation of B cells positively selected for high-affinity antibody.
) and loss of their association with BCL6. This leads to a failure to maintain silencing of these genes, which can now become reactivated even in the presence of BCL6. The double arrow between dark zone and light zone indicates that this is a reversible mechanism. This mechanism is rapid and may allow damaged B cells to be removed from the GC reaction. (C) In the second mechanism, sustained CD40 signaling, for example, by follicular dendritic cells, can activate NFκB, which in turn induces expression of IRF4, which can then directly repress BCL6 mRNA expression leading to down-regulation of BCL6 and up-regulation of its target genes. This mechanism is slower but is irreversible and leads to differentiation of B cells positively selected for high-affinity antibody.References
-
- Janeway CA, Jr, Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76:275–285. - PubMed
-
- Berek C, Ziegner M. The maturation of the immune response. Immunol Today. 1993;14:400–404. - PubMed
-
- Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 1997;276:589–592. - PubMed
-
- Ye BH, Cattoretti G, Shen Q, et al. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat Genet. 1997;16:161–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

