Targets of caspase-6 activity in human neurons and Alzheimer disease
- PMID: 18487604
- PMCID: PMC2500235
- DOI: 10.1074/mcp.M800007-MCP200
Targets of caspase-6 activity in human neurons and Alzheimer disease
Abstract
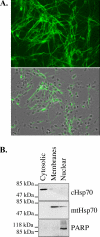
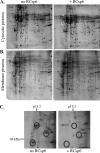
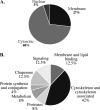
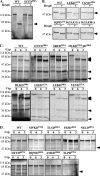
Caspase-6 activation occurs early in Alzheimer disease and sometimes precedes the clinical manifestation of the disease in aged individuals. The active Caspase-6 is localized in neuritic plaques, in neuropil threads, and in neurofibrillary tangles containing neurons that are not morphologically apoptotic in nature. To investigate the potential consequences of the activation of Caspase-6 in neurons, we conducted a proteomics analysis of Caspase-6-mediated cleavage of human neuronal proteins. Proteins from the cytosolic and membrane subcellular compartments were treated with recombinant active Caspase-6 and compared with undigested proteins by two-dimensional gel electrophoresis. LC/MS/MS analyses of the proteins that were cleaved identified 24 different potential protein substrates. Of these, 40% were cytoskeleton or cytoskeleton-associated proteins. We focused on the cytoskeleton proteins because these are critical for neuronal structure and function. Caspase-6 cleavage of alpha-Tubulin, alpha-Actinin-4, Spinophilin, and Drebrin was confirmed. At least one Caspase-6 cleavage site was identified for Drebrin, Spinophilin, and alpha-Tubulin. A neoepitope antiserum to alpha-Tubulin cleaved by Caspase-6 immunostained neurons, neurofibrillary tangles, neuropil threads, and neuritic plaques in Alzheimer disease and co-localized with active Caspase-6. These results imply that the early and neuritic activation of Caspase-6 in Alzheimer disease could disrupt the cytoskeleton network of neurons, resulting in impaired neuronal structure and function in the absence of cell death. This study provides novel insights into the pathophysiology of Alzheimer disease.
Figures


References
-
- Selznick, L., Holtzman, D., Han, B., Gokden, M., Srinivasan, A., Johnson, E., and Roth, K. ( 1999) In situ immunodetection of neuronal caspase-3 activation in Alzheimer's disease. J. Neuropathol. Exp. Neurol. 58, 1020–1026 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

