The C-terminal variable domain of LigB from Leptospira mediates binding to fibronectin
- PMID: 18487934
- PMCID: PMC2839090
- DOI: 10.4142/jvs.2008.9.2.133
The C-terminal variable domain of LigB from Leptospira mediates binding to fibronectin
Abstract
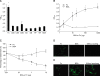
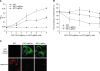
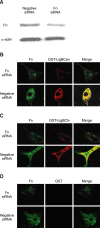
Adhesion through microbial surface components that recognize adhesive matrix molecules is an essential step in infection for most pathogenic bacteria. In this study, we report that LigB interacts with fibronectin (Fn) through its variable region. A possible role for LigB in bacterial attachment to host cells during the course of infection is supported by the following observations: (i) binding of the variable region of LigB to Madin-Darby canine kidney (MDCK) cells in a dose-dependent manner reduces the adhesion of Leptospira, (ii) inhibition of leptospiral attachment to Fn by the variable region of LigB, and (iii) decrease in binding of the variable region of LigB to the MDCK cells in the presence of Fn. Furthermore, we found a significant reduction in binding of the variable region of LigB to Fn using small interfering RNA (siRNA). Finally, the isothermal titration calorimetric results confirmed the interaction between the variable region of LigB and Fn. This is the first report to demonstrate that LigB binds to MDCK cells. In addition, the reduction of Fn expression in the MDCK cells, by siRNA, reduced the binding of LigB. Taken together, the data from the present study showed that LigB is a Fn-binding protein of pathogenic Leptospira spp. and may play a pivotal role in Leptospira-host interaction during the initial stage of infection.
Figures


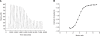
References
-
- Bulach DM, Zuerner RL, Wilson P, Seemann T, McGrath A, Cullen PA, Davis J, Johnson M, Kuczek E, Alt DP, Peterson-Burch B, Coppel RL, Rood JI, Davies JK, Adler B. Genome reduction in Leptospira borgpetersenii reflects limited transmission potential. Proc Natl Acad Sci USA. 2006;103:14560–14565. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

