Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies: application to GPCR oligomerization
- PMID: 18488035
- PMCID: PMC2642604
- DOI: 10.1038/nmeth.1213
Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies: application to GPCR oligomerization
Abstract
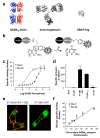
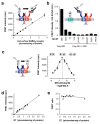
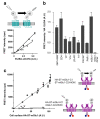
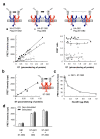
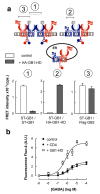
Cell-surface proteins are important in cell-cell communication. They assemble into heterocomplexes that include different receptors and effectors. Elucidation and manipulation of such protein complexes offers new therapeutic possibilities. We describe a methodology combining time-resolved fluorescence resonance energy transfer (FRET) with snap-tag technology to quantitatively analyze protein-protein interactions at the surface of living cells, in a high throughput-compatible format. Using this approach, we examined whether G protein-coupled receptors (GPCRs) are monomers or assemble into dimers or larger oligomers--a matter of intense debate. We obtained evidence for the oligomeric state of both class A and class C GPCRs. We also observed different quaternary structure of GPCRs for the neurotransmitters glutamate and gamma-aminobutyric acid (GABA): whereas metabotropic glutamate receptors assembled into strict dimers, the GABA(B) receptors spontaneously formed dimers of heterodimers, offering a way to modulate G-protein coupling efficacy. This approach will be useful in systematic analysis of cell-surface protein interaction in living cells.
Figures
References
-
- Bayburt TH, Leitz AJ, Xie G, Oprian DD, Sligar SG. Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J Biol Chem. 2007;282:14875–14881. - PubMed
-
- Chabre M, le Maire M. Monomeric G-protein-coupled receptor as a functional unit. Biochemistry. 2005;44:9395–9403. - PubMed

