The Ashwell receptor mitigates the lethal coagulopathy of sepsis
- PMID: 18488037
- PMCID: PMC2853759
- DOI: 10.1038/nm1760
The Ashwell receptor mitigates the lethal coagulopathy of sepsis
Abstract
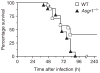
The Ashwell receptor, the major lectin of hepatocytes, rapidly clears from blood circulation glycoproteins bearing glycan ligands that include galactose and N-acetylgalactosamine. This asialoglycoprotein receptor activity remains a key factor in the development and administration of glycoprotein pharmaceuticals, yet a biological purpose of the Ashwell receptor has remained elusive. We have identified endogenous ligands of the Ashwell receptor as glycoproteins and regulatory components in blood coagulation and thrombosis that include von Willebrand factor (vWF) and platelets. The Ashwell receptor normally modulates vWF homeostasis and is responsible for thrombocytopenia during systemic Streptococcus pneumoniae infection by eliminating platelets desialylated by the bacterium's neuraminidase. Hemostatic adaptation by the Ashwell receptor moderates the onset and severity of disseminated intravascular coagulation during sepsis and improves the probability of host survival.
Figures


Comment in
-
Keeping blood clots at bay in sepsis.Nat Med. 2008 Jun;14(6):606-8. doi: 10.1038/nm0608-606. Nat Med. 2008. PMID: 18535572 No abstract available.
-
Hepatocytes clear platelets and are key regulators of disseminated intravascular coagulation during sepsis.J Hepatol. 2009 May;50(5):1060-1. doi: 10.1016/j.jhep.2009.02.008. Epub 2009 Mar 9. J Hepatol. 2009. PMID: 19328579 No abstract available.
References
-
- van den Hamer CJA, Morell AG, Scheinberg IH, Hickman J, Ashwell G. Galactosyl residues in the clearance of ceruloplasmin from the circulation. J Biol Chem. 1970;245:4397–4402. - PubMed
-
- Morell AG, Gregoriadis G, Scheinberg IH, Hickman J, Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971;246:1461–1467. - PubMed
-
- Ashwell G, Morell A. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol. 1974;41:99–128. - PubMed
-
- Hudgin RL, Pricer WE, Jr, Ashwell G, Stockert RJ, Morell AG. The isolation and properties of a rabbit liver binding protein specific for asialoglycoproteins. J Biol Chem. 1974;249:5536–5543. - PubMed
-
- Ashwell G, Kawasaki T. A protein from mammalian liver that specifically binds galactose-terminated glycoproteins. Methods Enzymol. 1978;50:287–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

