Renal hypoxia and dysoxia after reperfusion of the ischemic kidney
- PMID: 18488066
- PMCID: PMC2386087
- DOI: 10.2119/2008-00006.Legrand
Renal hypoxia and dysoxia after reperfusion of the ischemic kidney
Abstract
Ischemia is the most common cause of acute renal failure. Ischemic-induced renal tissue hypoxia is thought to be a major component in the development of acute renal failure in promoting the initial tubular damage. Renal oxygenation originates from a balance between oxygen supply and consumption. Recent investigations have provided new insights into alterations in oxygenation pathways in the ischemic kidney. These findings have identified a central role of microvascular dysfunction related to an imbalance between vasoconstrictors and vasodilators, endothelial damage and endothelium-leukocyte interactions, leading to decreased renal oxygen supply. Reduced microcirculatory oxygen supply may be associated with altered cellular oxygen consumption (dysoxia), because of mitochondrial dysfunction and activity of alternative oxygen-consuming pathways. Alterations in oxygen utilization and/or supply might therefore contribute to the occurrence of organ dysfunction. This view places oxygen pathways' alterations as a potential central player in the pathogenesis of acute kidney injury. Both in regulation of oxygen supply and consumption, nitric oxide seems to play a pivotal role. Furthermore, recent studies suggest that, following acute ischemic renal injury, persistent tissue hypoxia contributes to the development of chronic renal dysfunction. Adaptative mechanisms to renal hypoxia may be ineffective in more severe cases and lead to the development of chronic renal failure following ischemia-reperfusion. This paper is aimed at reviewing the current insights into oxygen transport pathways, from oxygen supply to oxygen consumption in the kidney and from the adaptation mechanisms to renal hypoxia. Their role in the development of ischemia-induced renal damage and ischemic acute renal failure are discussed.
Figures
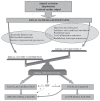


References
-
- Brady HR, Singer GG. Acute renal failure. Lancet. 1995;346:1533–40. - PubMed
-
- Bagshaw SM. The long-term outcome after acute renal failure. Curr Opin Crit Care. 2006;12:561–6. - PubMed
-
- Korkeila M, Ruokonen E, Takala J. Costs of care, long-term prognosis and quality of life in patients requiring renal replacement therapy during intensive care. Intensive Care Med. 2000;26:1824–31. - PubMed
-
- Manns B, et al. Cost of acute renal failure requiring dialysis in the intensive care unit: clinical and resource implications of renal recovery. Crit Care Med. 2003;31:449–55. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

