Systemic immune effects of adjuvant chemotherapy with 5-fluorouracil, epirubicin and cyclophosphamide and/or radiotherapy in breast cancer: a longitudinal study
- PMID: 18488220
- PMCID: PMC11030212
- DOI: 10.1007/s00262-008-0530-5
Systemic immune effects of adjuvant chemotherapy with 5-fluorouracil, epirubicin and cyclophosphamide and/or radiotherapy in breast cancer: a longitudinal study
Abstract
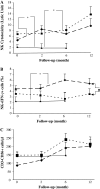
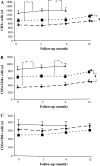
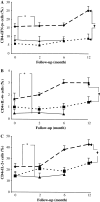
Immunotherapy is being increasingly utilized for adjuvant treatment for breast cancer (BC). We have previously described immune functions during primary therapy for BC. The present study describes immune recovery patterns during long-term, unmaintained follow-up after completion of adjuvant therapy.A group of patients with primary BC had been treated with adjuvant radio-chemotherapy (RT + CT) 5-fluorouracil, epirubicin and cyclophosphamide (FEC) (n = 21) and another group with radiotherapy (RT) (n = 20) alone. Immunological testing of NK and T-cell functions was performed initially at the end of adjuvant treatment and repeated after 2, 6 and 12 months. NK cell cytotoxicity was significantly higher (P < 0.05) at all time-points in patients than in age-matched controls and did not differ between the two treatments groups during one year observation. In contrast, lower numbers of CD4 T-cells and lower expression of CD28 on T-cells was observed particularly in RT + CT patients and did not normalize during the observation period. The numbers of T(reg) cells (CD4(+)CD25(high)) were low in the RT + CT group during follow-up, as well as expression of TCRxi, Zap70, p56(lck), P59(fyn) and PI3 k in CD4(+) cells. In contrast, expression of intracellular cytokines (IFN-gamma, IL-2, IL-4) in CD4 and CD8 T cells were significantly higher in RT + CT patients than in the RT group and the difference increased during follow-up. In conclusion, NK-cell cytotoxicity increased during unmaintained long-term follow-up whereas CD4 and regulatory T cells as well as signal transduction molecules remained low following adjuvant radio-chemotherapy.
Figures




Similar articles
-
Randomized trial comparing six versus three cycles of epirubicin-based adjuvant chemotherapy in premenopausal, node-positive breast cancer patients: 10-year follow-up results of the French Adjuvant Study Group 01 trial.J Clin Oncol. 2003 Jan 15;21(2):298-305. doi: 10.1200/JCO.2003.04.148. J Clin Oncol. 2003. PMID: 12525522 Clinical Trial.
-
Long-term cardiac follow-up in relapse-free patients after six courses of fluorouracil, epirubicin, and cyclophosphamide, with either 50 or 100 mg of epirubicin, as adjuvant therapy for node-positive breast cancer: French adjuvant study group.J Clin Oncol. 2004 Aug 1;22(15):3070-9. doi: 10.1200/JCO.2004.03.098. J Clin Oncol. 2004. PMID: 15284257 Clinical Trial.
-
Acute hematologic feasibility of G-CSF supported dose-escalated FEC therapy as adjuvant treatment after breast cancer surgery.Anticancer Res. 1999 Sep-Oct;19(5C):4429-34. Anticancer Res. 1999. PMID: 10650787
-
Final results of a randomized phase III trial comparing cyclophosphamide, epirubicin, and fluorouracil with a dose-intensified epirubicin and cyclophosphamide + filgrastim as neoadjuvant treatment in locally advanced breast cancer: an EORTC-NCIC-SAKK multicenter study.J Clin Oncol. 2003 Mar 1;21(5):843-50. doi: 10.1200/JCO.2003.05.135. J Clin Oncol. 2003. PMID: 12610183 Review.
-
Selection of adjuvant chemotherapy for treatment of node-positive breast cancer.Lancet Oncol. 2005 Nov;6(11):886-98. doi: 10.1016/S1470-2045(05)70424-1. Lancet Oncol. 2005. PMID: 16257797 Review.
Cited by
-
Trajectories of Stress, Depressive Symptoms, and Immunity in Cancer Survivors: Diagnosis to 5 Years.Clin Cancer Res. 2017 Jan 1;23(1):52-61. doi: 10.1158/1078-0432.CCR-16-0574. Epub 2016 Jul 12. Clin Cancer Res. 2017. PMID: 27407091 Free PMC article.
-
Relevance of tumor-infiltrating lymphocytes in breast cancer.BMC Med. 2015 Aug 24;13:202. doi: 10.1186/s12916-015-0431-3. BMC Med. 2015. PMID: 26300242 Free PMC article. Review.
-
Shifts in subsets of CD8+ T-cells as evidence of immunosenescence in patients with cancers affecting the lungs: an observational case-control study.BMC Cancer. 2015 Dec 28;15:1016. doi: 10.1186/s12885-015-2013-3. BMC Cancer. 2015. PMID: 26711627 Free PMC article.
-
Local High-Dose Radiotherapy Induces Systemic Immunomodulating Effects of Potential Therapeutic Relevance in Oligometastatic Breast Cancer.Front Immunol. 2017 Nov 6;8:1476. doi: 10.3389/fimmu.2017.01476. eCollection 2017. Front Immunol. 2017. PMID: 29163540 Free PMC article.
-
Immunomodulation of NK Cells by Ionizing Radiation.Front Oncol. 2020 Jun 16;10:874. doi: 10.3389/fonc.2020.00874. eCollection 2020. Front Oncol. 2020. PMID: 32612950 Free PMC article. Review.
References
-
- Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, Cabaret V, Fermeaux V, Bertheau P, Garnier J, Jeannin JF, Coudert B. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94:259–267. doi: 10.1038/sj.bjc.6602930. - DOI - PMC - PubMed
-
- Carson WE, Parihar R, Lindemann MJ, Personeni N, Dierksheide J, Meropol NJ, Baselga J, Caligiuri MA. Interleukin-2 enhances the natural killer cell response to Herceptin-coated Her2/neu-positive breast cancer cells. Eur J Immunol. 2001;31:3016–3025. doi: 10.1002/1521-4141(2001010)31:10<3016::AID-IMMU3016>3.0.CO;2-J. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

