Efficiency of adjuvant active specific immunization with Newcastle disease virus modified tumor cells in colorectal cancer patients following resection of liver metastases: results of a prospective randomized trial
- PMID: 18488223
- PMCID: PMC11030620
- DOI: 10.1007/s00262-008-0526-1
Efficiency of adjuvant active specific immunization with Newcastle disease virus modified tumor cells in colorectal cancer patients following resection of liver metastases: results of a prospective randomized trial
Abstract
Purpose: Metastatic disease is a major cause of mortality in colorectal cancer patients. Even after complete resection of isolated liver metastases, recurrence develops in the majority of patients. Therefore, development of strategies to prevent recurrent liver metastases is of major clinical importance. The present prospectively randomised phase III trial investigates the efficiency of active specific immunotherapy (ASI) after liver resection for hepatic metastases of colorectal cancer.
Methods: Patients with histologically confirmed liver metastases from colorectal cancer were randomised to the vaccination or control group. After complete resection of liver metastases, patients randomised to the vaccination group received six doses of Newcastle disease virus (NDV) infected autologous tumour cell vaccine (ATV-NDV). The primary end-point was overall survival, secondary end-points were disease-free survival and metastases-free survival.
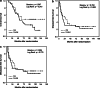
Results: Fifty-one patients were enrolled in the study with 50 patients available for analysis. The follow-up period was 116.1 +/- 23.8 month in the vaccination arm and 112.4 +/- 18.5 month in the control group. In the total patient group, no differences in the primary and secondary end-points were detected. Most interestingly, subgroup analysis revealed a significant advantage for vaccinated colon cancer patients with respect to overall survival [hazard ratio: 3.3; 95%, confidence interval (CI): 1.0-10.4; P = 0.042] and metastases-free survival (hazard ratio: 2.7; 95%, CI: 1.0-7.4; P = 0.047) in the intention-to-treat analysis.
Conclusion: Active specific immunotherapy in unselected colorectal cancer patients was not effective for prevention of recurrent metastatic disease. However, in colon cancer patients, ASI with ATV-NDV appears to be beneficial prolonging overall and metastases-free survival.
Figures


References
-
- Bengmark S, Hafstrom L. The natural history of primary and secondary malignant tumors of the liver, I. The prognosis for patients with hepatic metastases from colonic and rectal carcinoma by laparotomy. Cancer. 1969;23:198–202. doi: 10.1002/1097-0142(196901)23:1<198::AID-CNCR2820230126>3.0.CO;2-J. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

