Chemistry, biology, and medicinal potential of narciclasine and its congeners
- PMID: 18489166
- PMCID: PMC2856661
- DOI: 10.1021/cr078198u
Chemistry, biology, and medicinal potential of narciclasine and its congeners
Figures
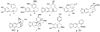
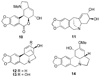
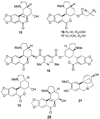
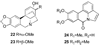

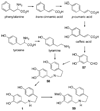
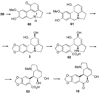
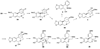
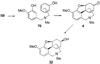
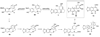
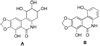
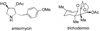
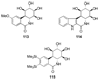
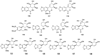

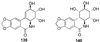
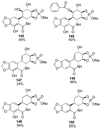
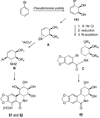
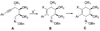
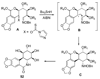
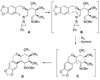
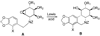
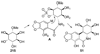
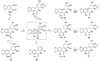
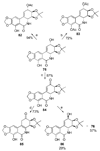
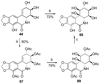
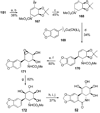
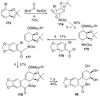
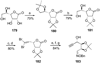
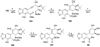
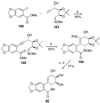

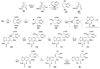
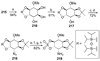
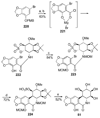
References
-
- Hartwell JL. Lloydia. 1967;30:379.
-
- Gardeil JB, translator. Traduction des Oeuvres Médicales d’Hippocrate. 4 vols. Toulouse: Fages, Meilhec et Cie; 1801.
-
- Aetios of Amida. The gynaecology and obsterics of the VI century, A.D. Philadelphia: Blakiston Co.; 1950. 215 pp. (transl. by J. V. Ricci)
-
- Leclerc L Ibn al-Baitar. Traité des Simples par Ibn el-Beïthar. I. Vol. 23. Paris: Notices et Extraits des Manuscrits de la Bibl. Nat.; 1877. 476 pp. 1881, 25(I), 489 pp.; 1883, 26(I), 483 pp.
-
- Martin SF. The Alkaloids. New York: Academic Press; 1987. p. 251.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

