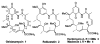Total synthesis of the Hsp90 inhibitor geldanamycin
- PMID: 18489177
- PMCID: PMC2430885
- DOI: 10.1021/ol800749w
Total synthesis of the Hsp90 inhibitor geldanamycin
Abstract
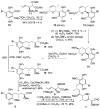
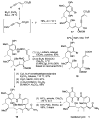
An enantioselective synthesis of the Hsp90 inhibitor geldanamycin was achieved in 20 linear steps and 2.0% overall yield from 2-methoxyhydroquinone. The synthesis is highlighted by a regio- and stereoselective hydroboration reaction; a Sc(OTf)(3)/Et(3)SiH-mediated pyran ring-opening reaction; an enantioselective crotylation to simultaneously install the C8-C9 (E) -trisubstituted olefin, the C10 and C11 stereocenters; a chelation-controlled asymmetric metallated acetylide addition; and an intramolecular copper(I)-mediated aryl amidation reaction to close the 19-membered macrolactam.
Figures
References
-
- Rutherford SL, Lindquist S. Nature. 1998;396:336. - PubMed
- Young JC, Moarefi I, Hartl FU. J Cell Biol. 2001;154:267. - PMC - PubMed
- Picard D. Cell Mol Life Sci. 2002;59:1640. - PMC - PubMed
- Le Brazidec JY, Kamal A, Busch D, Thao L, Zhang L, Timony G, Grecko R, Trent K, Lough R, Salazar T, Khan S, Burrows F, Boehm MF. J Med Chem. 2004;47 - PubMed
- Cheng H, Cao X, Xian M, Fang L, Cai TB, Ji JJ, Tunac JB, Sun D, Wang PG. J Med Chem. 2005;48:645. - PubMed
-
-
For syntheses of herbimycin, see: Nakata M, Osumi T, Ueno A, Kimura T, Tatsuta K. Tetrahedron Lett. 1991;32:6015.Nakata M, Osumi T, Ueno A, Kimura T, Tatsuta K. Bull Chem Soc Jpn. 1992;65:2974.Carter KD, Panek JS. Org Lett. 2004;6:55.Canova S, Bellosta V, Bigot A, Mailliet P, Mignani S, Cossy J. Org Lett. 2007;9:145.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous