Redundancy renders the glycoprotein 96 receptor scavenger receptor A dispensable for cross priming in vivo
- PMID: 18489571
- PMCID: PMC2612544
- DOI: 10.1111/j.1365-2567.2008.02861.x
Redundancy renders the glycoprotein 96 receptor scavenger receptor A dispensable for cross priming in vivo
Abstract
CD8(+) T cells (T(CD8+)) differentiate into effector cells following recognition of specific peptide-major histocompatibility complex (MHC) class I complexes (pMHC-I) on the surface of professional APCs (pAPCs), such as dendritic cells. Antigenic pMHC-I can be generated from two spatially distinct sources. The direct presentation pathway involves generation of peptide from protein substrate synthesized within the cell that is presenting the pMHC-I. Alternatively, the cross presentation pathway involves presentation of antigen that is not synthesized within the presenting cell, but is derived from exogenous proteins synthesized within other donor cells. The mechanisms by which cross presentation of exogenous antigens occur in vivo remain controversial. The C-type lectin scavenger receptor A (SR-A) has been implicated in a number of potential cross presentation pathways, including the presentation of peptide bound to heat shock proteins, such as glycoprotein 96 (gp96), and the transfer of pMHC-I from a donor cell to the pAPC. We demonstrate here that initiation of T(CD8+) responses is normal in mice lacking SR-A, and that the redundancy of ligand binding exhibited by the SR family is likely to be an important mechanism that ensures cross presentation in vivo. These observations emphasize the requirement to target multiple receptors and antigen-processing pathways during the rational design of vaccines aimed at eliciting protective T(CD8+).
Figures
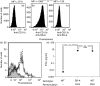

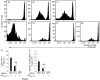
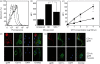
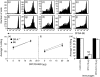
Similar articles
-
Multiple antigen-specific processing pathways for activating naive CD8+ T cells in vivo.J Immunol. 2001 Apr 1;166(7):4355-62. doi: 10.4049/jimmunol.166.7.4355. J Immunol. 2001. PMID: 11254689
-
Viral sequestration of antigen subverts cross presentation to CD8(+) T cells.PLoS Pathog. 2009 May;5(5):e1000457. doi: 10.1371/journal.ppat.1000457. Epub 2009 May 29. PLoS Pathog. 2009. PMID: 19478869 Free PMC article.
-
Peptide-MHC-I from Endogenous Antigen Outnumber Those from Exogenous Antigen, Irrespective of APC Phenotype or Activation.PLoS Pathog. 2015 Jun 24;11(6):e1004941. doi: 10.1371/journal.ppat.1004941. eCollection 2015 Jun. PLoS Pathog. 2015. PMID: 26107264 Free PMC article.
-
Cross-presentation of Exogenous Antigens.Transfus Clin Biol. 2019 Nov;26(4):346-351. doi: 10.1016/j.tracli.2019.01.006. Epub 2019 Feb 1. Transfus Clin Biol. 2019. PMID: 30797678 Review.
-
Endocytic Recycling of MHC Class I Molecules in Non-professional Antigen Presenting and Dendritic Cells.Front Immunol. 2019 Jan 7;9:3098. doi: 10.3389/fimmu.2018.03098. eCollection 2018. Front Immunol. 2019. PMID: 30666258 Free PMC article. Review.
Cited by
-
A marked reduction in priming of cytotoxic CD8+ T cells mediated by stress-induced glucocorticoids involves multiple deficiencies in cross-presentation by dendritic cells.J Immunol. 2011 Jan 1;186(1):183-94. doi: 10.4049/jimmunol.1001737. Epub 2010 Nov 22. J Immunol. 2011. PMID: 21098225 Free PMC article.
-
Efficient cross-priming of antiviral CD8+ T cells by antigen donor cells is GRP94 independent.J Immunol. 2009 Oct 1;183(7):4205-10. doi: 10.4049/jimmunol.0901828. Epub 2009 Sep 14. J Immunol. 2009. PMID: 19752220 Free PMC article.
-
Interaction of Toll-Like Receptors with the Molecular Chaperone Gp96 Is Essential for Its Activation of Cytotoxic T Lymphocyte Response.PLoS One. 2016 May 16;11(5):e0155202. doi: 10.1371/journal.pone.0155202. eCollection 2016. PLoS One. 2016. PMID: 27183126 Free PMC article.
-
Heat Shock Proteins 90 kDa: Immunomodulators and Adjuvants in Vaccine Design Against Infectious Diseases.Front Bioeng Biotechnol. 2021 Jan 20;8:622186. doi: 10.3389/fbioe.2020.622186. eCollection 2020. Front Bioeng Biotechnol. 2021. PMID: 33553125 Free PMC article. Review.
-
Heat Shock Protein 90's Mechanistic Role in Contact Hypersensitivity.J Immunol. 2022 Jun 15;208(12):2622-2631. doi: 10.4049/jimmunol.2101023. Epub 2022 Jun 8. J Immunol. 2022. PMID: 35675957 Free PMC article.
References
-
- Norbury CC, Basta S, Donohue KB, et al. CD8+ T cell cross-priming via transfer of proteasome substrates. Science. 2004;304:1318–21. - PubMed
-
- Wolkers MC, Brouwenstijn N, Bakker AH, Toebes M, Schumacher TN. Antigen bias in T cell cross-priming. Science. 2004;304:1314–7. - PubMed
-
- Basta S, Stoessel R, Basler M, van den Broek M, Groettrup M. Cross-presentation of the long-lived lymphocytic choriomeningitis virus nucleoprotein does not require neosynthesis and is enhanced via heat shock proteins. J Immunol. 2005;175:796–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- C06 RR-15428-01/RR/NCRR NIH HHS/United States
- P20 RR016437/RR/NCRR NIH HHS/United States
- CA60395-11/CA/NCI NIH HHS/United States
- T32 CA060395/CA/NCI NIH HHS/United States
- AI056094/AI/NIAID NIH HHS/United States
- C06 RR015428/RR/NCRR NIH HHS/United States
- AI070537/AI/NIAID NIH HHS/United States
- CA-102392/CA/NCI NIH HHS/United States
- R01 AI070537/AI/NIAID NIH HHS/United States
- R56 AI056094/AI/NIAID NIH HHS/United States
- R01 AI067405/AI/NIAID NIH HHS/United States
- R01 AI056094/AI/NIAID NIH HHS/United States
- AI067405/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

