Oral probiotic and prevention of Pseudomonas aeruginosa infections: a randomized, double-blind, placebo-controlled pilot study in intensive care unit patients
- PMID: 18489775
- PMCID: PMC2481460
- DOI: 10.1186/cc6907
Oral probiotic and prevention of Pseudomonas aeruginosa infections: a randomized, double-blind, placebo-controlled pilot study in intensive care unit patients
Abstract
Introduction: Preventing carriage of potentially pathogenic micro-organisms from the aerodigestive tract is an infection control strategy used to reduce the occurrence of ventilator-associated pneumonia in intensive care units. However, antibiotic use in selective decontamination protocols is controversial. The purpose of this study was to investigate the effect of oral administration of a probiotic, namely Lactobacillus, on gastric and respiratory tract colonization/infection with Pseudomonas aeruginosa strains. Our hypothesis was that an indigenous flora should exhibit a protective effect against secondary colonization.
Methods: We conducted a prospective, randomized, double-blind, placebo-controlled pilot study between March 2003 and October 2004 in a 17-bed intensive care unit of a teaching hospital in Clermont-Ferrand, France. Consecutive patients with a unit stay of longer than 48 hours were included, 106 in the placebo group and 102 in the probiotic group. Through a nasogastric feeding tube, patients received either 109 colony-forming units unity forming colony of Lactobacillus casei rhamnosus or placebo twice daily, from the third day after admission to discharge. Digestive tract carriage of P. aeruginosa was monitored by cultures of gastric aspirates at admission, once a week thereafter and on discharge. In addition, bacteriological analyses of respiratory tract specimens were conducted to determine patient infectious status.
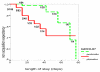
Results: The occurrence of P. aeruginosa respiratory colonization and/or infection was significantly delayed in the probiotic group, with a difference in median delay to acquisition of 11 days versus 50 days (P = 0.01), and a nonacquisition expectancy mean of 69 days versus 77 days (P = 0.01). The occurrence of ventilator-associated pneumonia due to P. aeruginosa in the patients receiving the probiotic was less frequent, although not significantly reduced, in patients in the probiotic group (2.9%) compared with those in the placebo group (7.5%). After multivariate Cox proportional hazards modelling, the absence of probiotic treatment increased the risk for P. aeruginosa colonization in respiratory tract (adjusted hazard ratio = 3.2, 95% confidence interval - 1.1 to 9.1).
Conclusion: In this pilot study, oral administration of a probiotic delayed respiratory tract colonization/infection by P. aeruginosa.
Trial registration: The trial registration number for this study is NCT00604110.
Figures
Comment in
-
Probiotics in the intensive care unit: why controversies and confusion abound.Crit Care. 2008;12(3):160. doi: 10.1186/cc6927. Epub 2008 Jun 24. Crit Care. 2008. PMID: 18598379 Free PMC article.
References
-
- Bert F, Lambert-Zechovsky N. Bacteria isolated from protected bronchopulmonary samples: variation as a function of the previous length of stay in the recovery room. Pathol Biol. 1998;46:380–384. - PubMed
-
- Bonten MJ, Gaillard CA, Hulst R van der, de Leeuw PW, Geest S van der, Stobberingh EE, Soeters PB. Intermittent enteral feeding: the influence on respiratory and digestive tract colonization in mechanically ventilated intensive-care-unit patients. Am J Respir Crit Care Med. 1996;154:394–399. - PubMed
-
- Bergmans DC, Bonten MJ, Gaillard CA, Paling JC, Geest S van der, van Tiel FH, Beysens AJ, de Leeuw PW, Stobberingh EE. Prevention of ventilator-associated pneumonia by oral decontamination: a prospective, randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med. 2001;164:382–388. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical