Tolerance and rebound with zafirlukast in patients with persistent asthma
- PMID: 18489783
- PMCID: PMC2426667
- DOI: 10.1186/1477-5751-7-3
Tolerance and rebound with zafirlukast in patients with persistent asthma
Abstract
Background: The potential for tolerance to develop to zafirlukast, a cysteinyl leukotriene (CysLT) receptor antagonist (LRA) in persistent asthma, has not been specifically examined.
Objective: To look for any evidence of tolerance and potential for short-term clinical worsening on LRA withdrawal. Outcome measures included changes in; airway hyperresponsiveness to inhaled methacholine (PD20FEV1), daily symptoms and peak expiratory flows (PEF), sputum and blood cell profiles, sputum CysLT and prostaglandin (PG)E2 and exhaled nitric oxide (eNO) levels.
Methods: A double blind, placebo-controlled study of zafirlukast, 20 mg twice daily over 12 weeks in 21 asthmatics taking beta2-agonists only (Group I), and 24 subjects treated with ICS (Group II).
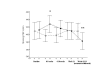
Results: In Group I, zafirlukast significantly improved morning PEF and FEV1compared to placebo (p < 0.01), and reduced morning waking with asthma from baseline after two weeks (p < 0.05). Similarly in Group II, FEV1 improved compared to placebo (p < 0.05), and there were early within-treatment group improvements in morning PEF, beta2-agonist use and asthma severity scores (p < 0.05). However, most improvements with zafirlukast in Group I and to a lesser extent in Group II deteriorated toward baseline values over 12 weeks. In both groups, one week following zafirlukast withdrawal there were significant deteriorations in morning and evening PEFs and FEV1 compared with placebo (p < or = 0.05) and increased nocturnal awakenings in Group II (p < 0.05). There were no changes in PD20FEV1, sputum CysLT concentrations or exhaled nitric oxide (eNO) levels. However, blood neutrophils significantly increased in both groups following zafirlukast withdrawal compared to placebo (p = 0.007).
Conclusion: Tolerance appears to develop to zafirlukast and there is rebound clinical deterioration on drug withdrawal, accompanied by a blood neutrophilia.
Figures
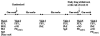
Similar articles
-
Therapeutic effect of zafirlukast as monotherapy in steroid-naive patients with severe persistent asthma.Chest. 1999 Feb;115(2):336-42. doi: 10.1378/chest.115.2.336. Chest. 1999. PMID: 10027429 Clinical Trial.
-
Effects of adding a leukotriene antagonist or a long-acting beta(2)-agonist in asthmatic patients with the glycine-16 beta(2)-adrenoceptor genotype.Am J Med. 2000 Aug 1;109(2):114-21. doi: 10.1016/s0002-9343(00)00454-x. Am J Med. 2000. PMID: 10967152 Clinical Trial.
-
The effect of low-dose inhaled fluticasone propionate on exhaled nitric oxide in asthmatic patients and comparison with oral zafirlukast.Ann Allergy Asthma Immunol. 2001 Oct;87(4):283-8. doi: 10.1016/S1081-1206(10)62241-7. Ann Allergy Asthma Immunol. 2001. PMID: 11686419 Clinical Trial.
-
Zafirlukast: an update of its pharmacology and therapeutic efficacy in asthma.Drugs. 2001;61(2):285-315. doi: 10.2165/00003495-200161020-00012. Drugs. 2001. PMID: 11270943 Review.
-
Zafirlukast. A review of its pharmacology and therapeutic potential in the management of asthma.Drugs. 1998 Jan;55(1):121-44. doi: 10.2165/00003495-199855010-00008. Drugs. 1998. PMID: 9463793 Review.
Cited by
-
Leukotriene-receptor antagonists versus placebo in the treatment of asthma in adults and adolescents: a systematic review and meta-analysis.Ann Intern Med. 2015 Nov 17;163(10):756-67. doi: 10.7326/M15-1059. Epub 2015 Sep 22. Ann Intern Med. 2015. PMID: 26390230 Free PMC article.
-
Does zafirlukast reduce future risk of asthma exacerbations in adults? Systematic review and meta-analysis.Multidiscip Respir Med. 2014 May 28;9(1):30. doi: 10.1186/2049-6958-9-30. eCollection 2014. Multidiscip Respir Med. 2014. PMID: 24936302 Free PMC article. Review.
-
Which biomarkers are effective for identifying Th2-driven inflammation in asthma?Curr Allergy Asthma Rep. 2013 Oct;13(5):477-86. doi: 10.1007/s11882-013-0376-6. Curr Allergy Asthma Rep. 2013. PMID: 23918590 Review.
References
-
- Leff AR. Discovery of leukotrienes and development of antileukotriene agents. Ann Allergy Asthma Immunol. 2001;86:4–8. - PubMed
-
- Busse W. The role and contribution of leukotrienes in asthma. Ann Allergy Asthma Immunol. 1998;81:17–26; quiz 26-9. - PubMed
-
- Devillier P, Bessard G, Advenier C. [Leukotriene antagonists: a new approach in the treatment of asthma] Rev Mal Respir. 1997;14:159–170. - PubMed
-
- Christian Virchow J, Prasse A, Naya I, Summerton L, Harris A. Zafirlukast improves asthma control in patients receiving high-dose inhaled corticosteroids. Am J Respir Crit Care Med. 2000;162:578–585. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

