Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu) compares favorably with i.v. busulfan and cyclophosphamide (i.v. BuCy2) as pretransplant conditioning therapy in AML/MDS
- PMID: 18489993
- PMCID: PMC4230823
- DOI: 10.1016/j.bbmt.2008.03.009
Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu) compares favorably with i.v. busulfan and cyclophosphamide (i.v. BuCy2) as pretransplant conditioning therapy in AML/MDS
Abstract
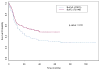
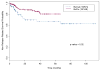
We postulated that fludarabine (Flu) instead of cyclophosphamide (Cy) combined with i.v. busulfan (Bu) as preconditioning for allogeneic hematopoietic stem cell transplantation (HSCT) would improve safety and retain antileukemic efficacy. Sixty-seven patients received BuCy2, and subsequently, 148 patients received Bu-Flu. We used a Bayesian method to compare outcomes between these nonrandomized patients. The groups had comparable pretreatment characteristics, except that Bu-Flu patients were older (46 versus 39 years, P < .01), more often had unrelated donors (47.3% versus 20.9%, P < .0003), and had shorter median follow-up (39.7 versus 74.6 months). To account for improved supportive care and other unidentified factors that may affect outcome ("period" effects), 78 acute myelogenous leukemia (AML) patients receiving Melphalan-Flu (MF), treated in parallel during this time (1997-2004) were used to estimate the period effect. The MF patients' outcomes worsened during this period. Therefore, the period effect is unlikely to explain the greatly improved outcome with Bu-Flu. Patients transplanted with Bu-Flu in the first complete remission (CR1) had a 3-year overall survival and event-free-survival (EFS) of 78% and 74%, respectively, whereas CR1 patients younger than age 41 had a 3-year EFS of 83%. These results support replacing BuCy +/- ATG with Bu-Flu +/- rabbit-antithymocyte globulin (ATG), and warrant a prospective comparison between allogeneic HSCT and conventional induction/consolidation chemotherapy for AML in CR1.
Figures





References
-
- Bhagwatwar HP, Phadungpojna S, Chow DS, Andersson BS. Formulation and stability of busulfan for intravenous administration in high-dose chemotherapy. Cancer Chemother Pharmacol. 1996;37:401–408. - PubMed
-
- Thall PF, Champlin RE, Andersson BS. Comparison of 100-day mortality rates associated with i.v. busulfan and cyclophosphamide vs other preparative regimens in allogeneic bone marrow transplantation for chronic myelogenous leukemia: Bayesian sensitivity analyses of confounded treatment and center effects. Bone Marrow Transplant. 2004;33:1191–1199. - PubMed
-
- de Lima M, Anagnostopoulous A, Munsell M, et al. Non-Ablative versus Reduced Intensity Conditioning Regimens in the treatment of Acute Myeloid Leukemia and High-Risk Myelodysplastic Syndrome. Dose is Relevant for Long-Term Disease Control after Allogeneic Hematopoietic Stem Cell Transplantation. Blood. 2004;104 :865–872. - PubMed
-
- Shimoni A, Hardan I, Shem-Tov N, Yeshurun M, Yerushalmi R, Avigdor A, Ben-Bassat I, Nagler A. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia. 2006;20:322–328. - PubMed
-
- Shimoni A, Hardan I, Shem-Tov N, et al. Comparison between two fludarabine-based reduced-intensity conditioning regimens before allogeneic hematopoietic stem-cell transplantation: fludarabine/Melphalan is associated with higher incidence of acute graft-versus-host disease and non-relapse mortality and lower incidence of relapse than fludarabine/busulfan. Leukemia. 2007;21:2109–2116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

