Structural polymorphism within a regulatory element of the human KRAS promoter: formation of G4-DNA recognized by nuclear proteins
- PMID: 18490377
- PMCID: PMC2441797
- DOI: 10.1093/nar/gkn120
Structural polymorphism within a regulatory element of the human KRAS promoter: formation of G4-DNA recognized by nuclear proteins
Abstract
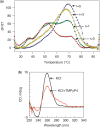
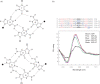
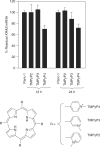
The human KRAS proto-oncogene contains a critical nuclease hypersensitive element (NHE) upstream of the major transcription initiation site. In this article, we demonstrate by primer-extension experiments, PAGE, chemical footprinting, CD, UV and FRET experiments that the G-rich strand of NHE (32R) folds into intra-molecular G-quadruplex structures. Fluorescence data show that 32R in 100 mM KCl melts with a biphasic profile, showing the formation of two distinct G-quadruplexes with T(m) of approximately 55 degrees C (Q(1)) and approximately 72 degrees C (Q(2)). DMS-footprinting and CD suggest that Q(1) can be a parallel and Q(2) a mixed parallel/antiparallel G-quadruplex. When dsNHE (32R hybridized to its complementary) is incubated with a nuclear extract from Panc-1 cells, three DNA-protein complexes are observed by EMSA. The complex of slower mobility is competed by quadruplex 32R, but not by mutant oligonucleotides, which cannot form a quadruplex structure. Using paramagnetic beads coupled with 32R, we pulled down from the Panc-1 extract proteins with affinity for quadruplex 32R. One of these is the heterogeneous nuclear ribonucleoprotein A1, which was previously reported to unfold quadruplex DNA. Our study suggests a role of quadruplex DNA in KRAS transcription and provides the basis for the rationale design of molecular strategies to inhibit the expression of KRAS.
Figures






Similar articles
-
Protein hnRNP A1 and its derivative Up1 unfold quadruplex DNA in the human KRAS promoter: implications for transcription.Nucleic Acids Res. 2009 May;37(9):2841-53. doi: 10.1093/nar/gkp138. Epub 2009 Mar 12. Nucleic Acids Res. 2009. PMID: 19282454 Free PMC article.
-
Protein hnRNPA1 binds to a critical G-rich element of KRAS and unwinds G-quadruplex structures: implications in transcription.Nucleic Acids Symp Ser (Oxf). 2008;(52):159-60. doi: 10.1093/nass/nrn081. Nucleic Acids Symp Ser (Oxf). 2008. PMID: 18776302
-
G-quadruplex formation within the promoter of the KRAS proto-oncogene and its effect on transcription.Nucleic Acids Res. 2006 May 10;34(9):2536-49. doi: 10.1093/nar/gkl286. Print 2006. Nucleic Acids Res. 2006. PMID: 16687659 Free PMC article.
-
Critical role of hnRNP A1 in activating KRAS transcription in pancreatic cancer cells: A molecular mechanism involving G4 DNA.Biochim Biophys Acta Gen Subj. 2017 May;1861(5 Pt B):1389-1398. doi: 10.1016/j.bbagen.2016.11.031. Epub 2016 Nov 22. Biochim Biophys Acta Gen Subj. 2017. PMID: 27888145 Review.
-
G4 DNA in ras genes and its potential in cancer therapy.Biochim Biophys Acta. 2016 Apr;1859(4):663-74. doi: 10.1016/j.bbagrm.2016.02.002. Epub 2016 Feb 4. Biochim Biophys Acta. 2016. PMID: 26855080 Review.
Cited by
-
Structural insight into the bulge-containing KRAS oncogene promoter G-quadruplex bound to berberine and coptisine.Nat Commun. 2022 Oct 12;13(1):6016. doi: 10.1038/s41467-022-33761-4. Nat Commun. 2022. PMID: 36224201 Free PMC article.
-
Switching G-quadruplex to parallel duplex by molecular rotor clustering.Nucleic Acids Res. 2022 Oct 14;50(18):10249-10263. doi: 10.1093/nar/gkac811. Nucleic Acids Res. 2022. PMID: 36130267 Free PMC article.
-
Profiling of i-motif-binding proteins reveals functional roles of nucleolin in regulation of high-order DNA structures.Nucleic Acids Res. 2024 Dec 11;52(22):13530-13543. doi: 10.1093/nar/gkae1001. Nucleic Acids Res. 2024. PMID: 39557413 Free PMC article.
-
Non-duplex G-Quadruplex Structures Emerge as Mediators of Epigenetic Modifications.Trends Genet. 2019 Feb;35(2):129-144. doi: 10.1016/j.tig.2018.11.001. Epub 2018 Dec 4. Trends Genet. 2019. PMID: 30527765 Free PMC article. Review.
-
DNA G-Quadruplexes as Targets for Natural Product Drug Discovery.Engineering (Beijing). 2024 Jul;38:39-51. doi: 10.1016/j.eng.2024.03.015. Epub 2024 May 6. Engineering (Beijing). 2024. PMID: 40809342 Free PMC article.
References
-
- Barbacid M. Ras genes. Annu. Rev. Biochem. 1987;56:779–827. - PubMed
-
- Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat. Rev. Cancer. 2003;3:459–465. - PubMed
-
- Yanes L, Groffen J, Valenzuela DM. cKRAS mutations in human carcinomas occur preferentially in codon 12. Oncogene. 1987;1:315–318. - PubMed
-
- Nagata Y, Abe M, Kobayashi K, Yoshida K, Ishibashi T, Naoe T, Nakayama E, Shiku H. Glycine to aspartic acid mutations at codon 13 of the c-Ki-ras gene in human gastrointestinal cancers. Cancer Res. 1990;50:480–482. - PubMed
-
- Adjei AA. Blocking oncogenic Ras signaling for cancer therapy. J. Natl Cancer. Inst. 2002;94:1031–1032. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

