Aspergillus fumigatus stimulates leukocyte adhesion molecules and cytokine production by endothelial cells in vitro and during invasive pulmonary disease
- PMID: 18490455
- PMCID: PMC2493209
- DOI: 10.1128/IAI.01510-07
Aspergillus fumigatus stimulates leukocyte adhesion molecules and cytokine production by endothelial cells in vitro and during invasive pulmonary disease
Abstract
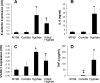
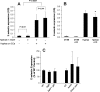
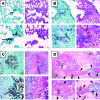
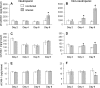
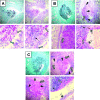
Invasive aspergillosis is characterized by hyphal invasion of the blood vessels, which contributes to the pathogenesis of this disease. During this angioinvasion, Aspergillus fumigatus interacts with the endothelial cell lining of the blood vessels. We investigated the response of vascular endothelial cells to A. fumigatus infection in vitro and in mouse models of invasive pulmonary aspergillosis. Infection with hyphae, but not with conidia, stimulated endothelial cells to synthesize E-selectin, vascular cell adhesion molecule 1 (VCAM-1), interleukin 8, and tumor necrosis factor alpha (TNF-alpha) in vitro. Killed hyphae induced approximately 40% less stimulation than did live hyphae. Endothelial cell stimulation required contact between the hyphae and endothelial cells but not endocytosis of the organisms. Studies with DeltagliP and DeltastuA null mutants of A. fumigatus indicated that the extent of endothelial cell stimulation was not influenced by gliotoxin or other StuA-dependent factors synthesized by A. fumigatus. In neutropenic mice infected with wild-type A. fumigatus, increased pulmonary expression of E-selectin, cytokine-induced neutrophil chemoattractant (KC), and TNF-alpha occurred only when neutropenia had resolved. In nonneutropenic mice immunosuppressed with corticosteroids, A. fumigatus stimulated earlier pulmonary expression of E-selectin, VCAM-1, and KC, while expression of intercellular adhesion molecule 1 and TNF-alpha was suppressed. In both mouse models, expression of E-selectin and KC was associated with high pulmonary fungal burden, angioinvasion, and neutrophil adherence to endothelial cells. Therefore, the expression of leukocyte adhesion molecules and secretion of proinflammatory cytokines by endothelial cells in response to A. fumigatus could enhance the host defense against this organism by contributing to the recruitment of activated leukocytes to sites of angioinvasion.
Figures
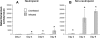
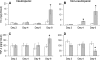
Similar articles
-
Interactions of Aspergillus fumigatus with vascular endothelial cells.Med Mycol. 2006 Sep;44 Suppl 1:S115-7. doi: 10.1080/13693780600897989. Med Mycol. 2006. PMID: 17050430
-
Polarized response of endothelial cells to invasion by Aspergillus fumigatus.Cell Microbiol. 2009 Jan;11(1):170-82. doi: 10.1111/j.1462-5822.2008.01247.x. Epub 2008 Oct 30. Cell Microbiol. 2009. PMID: 19016788 Free PMC article.
-
Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus.J Immunol. 2002 Feb 1;168(3):1362-71. doi: 10.4049/jimmunol.168.3.1362. J Immunol. 2002. PMID: 11801677
-
Pulmonary defense mechanisms against opportunistic fungal pathogens.Immunol Ser. 1989;47:243-71. Immunol Ser. 1989. PMID: 2490078 Review.
-
Immune responses to Aspergillus fumigatus infections.Biol Blood Marrow Transplant. 2006 Jan;12(1 Suppl 1):47-9. doi: 10.1016/j.bbmt.2005.09.007. Biol Blood Marrow Transplant. 2006. PMID: 16399584 Review.
Cited by
-
Innate immunity to Aspergillus species.Clin Microbiol Rev. 2009 Oct;22(4):535-51. doi: 10.1128/CMR.00014-09. Clin Microbiol Rev. 2009. PMID: 19822887 Free PMC article. Review.
-
AtrR Is an Essential Determinant of Azole Resistance in Aspergillus fumigatus.mBio. 2019 Mar 12;10(2):e02563-18. doi: 10.1128/mBio.02563-18. mBio. 2019. PMID: 30862750 Free PMC article.
-
Role of trehalose biosynthesis in Aspergillus fumigatus development, stress response, and virulence.Infect Immun. 2010 Jul;78(7):3007-18. doi: 10.1128/IAI.00813-09. Epub 2010 May 3. Infect Immun. 2010. PMID: 20439478 Free PMC article.
-
Gene expression profiles of human dendritic cells interacting with Aspergillus fumigatus in a bilayer model of the alveolar epithelium/endothelium interface.PLoS One. 2014 May 28;9(5):e98279. doi: 10.1371/journal.pone.0098279. eCollection 2014. PLoS One. 2014. PMID: 24870357 Free PMC article.
-
Functional convergence of gliP and aspf1 in Aspergillus fumigatus pathogenicity.Virulence. 2018;9(1):1062-1073. doi: 10.1080/21505594.2018.1482182. Virulence. 2018. PMID: 30052103 Free PMC article.
References
-
- Cornillet, A., C. Camus, S. Nimubona, V. Gandemer, P. Tattevin, C. Belleguic, S. Chevrier, C. Meunier, C. Lebert, M. Aupee, S. Caulet-Maugendre, M. Faucheux, B. Lelong, E. Leray, C. Guiguen, and J. P. Gangneux. 2006. Comparison of epidemiological, clinical, and biological features of invasive aspergillosis in neutropenic and nonneutropenic patients: a 6-year survey. Clin. Infect. Dis. 43577-584. - PubMed
-
- Denning, D. W. 2000. Aspergillus species, p. 2674-2684. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 5th ed. Churchill Livingston, Philadelphia, PA.
-
- Duong, M., N. Ouellet, M. Simard, Y. Bergeron, M. Olivier, and M. G. Bergeron. 1998. Kinetic study of host defense and inflammatory response to Aspergillus fumigatus in steroid-induced immunosuppressed mice. J. Infect. Dis. 1781472-1482. - PubMed
-
- Galley, H. F., and N. R. Webster. 2004. Physiology of the endothelium. Br. J. Anaesth. 93105-113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

