Yersinia pestis type III secretion system-dependent inhibition of human polymorphonuclear leukocyte function
- PMID: 18490459
- PMCID: PMC2493194
- DOI: 10.1128/IAI.00385-08
Yersinia pestis type III secretion system-dependent inhibition of human polymorphonuclear leukocyte function
Abstract
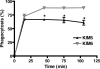
Human polymorphonuclear leukocytes (PMNs, or neutrophils) are the primary innate host defense against invading bacterial pathogens. Neutrophils are rapidly recruited to sites of infection and ingest microorganisms through a process known as phagocytosis. Following phagocytosis by human PMNs, microorganisms are killed by reactive oxygen species (ROS) and microbicidal products contained within granules. Yersinia pestis, the causative agent of plague, is capable of rapid replication and dissemination from sites of infection in the host. Although Y. pestis survives in macrophages, the bacterial fate following interaction with human PMNs is less clear. The ability of Y. pestis to inhibit phagocytosis by human PMNs was assessed by differential fluorescence microscopy and was shown to be dependent on expression of the type III secretion system (TTSS). Previous studies have demonstrated that TTSS expression in enteropathogenic Yersinia spp. also inhibits the respiratory burst in PMNs and macrophages, and we show here that human PMN ROS production is similarly repressed by Y. pestis. However, exclusion of uningested TTSS-expressing Y. pestis with gentamicin revealed that intracellular bacteria are eliminated by human PMNs, similar to bacteria lacking the TTSS. In summary, our results suggest that the Y. pestis TTSS contributes to extracellular survival following interactions with human PMNs and that the intracellular fate is independent of TTSS inhibition of neutrophil ROS production.
Figures

References
-
- Allen, L. A. 2003. Mechanisms of pathogenesis: evasion of killing by polymorphonuclear leukocytes. Microbes Infect. 51329-1335. - PubMed
-
- Allen, L. A., B. R. Beecher, J. T. Lynch, O. V. Rohner, and L. M. Wittine. 2005. Helicobacter pylori disrupts NADPH oxidase targeting in human neutrophils to induce extracellular superoxide release. J. Immunol. 1743658-3667. - PubMed
-
- Bengoechea, J., B. Lidner, U. Seydel, R. Diaz, and I. Moriyon. 1998. Yersinia pseudotuberculosis and Yersinia pestis are more resistant to bacterial cationic peptides than Yersinia enterocolitica. Microbiology 1441509-1515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

