CD43 regulates Th2 differentiation and inflammation
- PMID: 18490738
- PMCID: PMC2669414
- DOI: 10.4049/jimmunol.180.11.7385
CD43 regulates Th2 differentiation and inflammation
Abstract
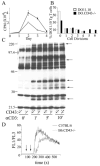
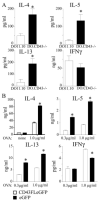
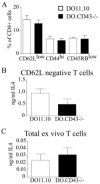
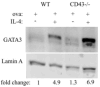
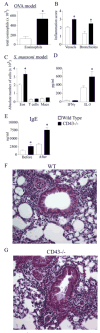
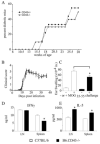
CD43 is a highly glycosylated transmembrane protein that regulates T cell activation. CD43(-/-) T cells are hyperproliferative and the cytoplasmic tail of CD43 has been found to be sufficient to reconstitute wild-type proliferation levels, suggesting an intracellular mechanism. In this study, we report that upon TCR ligation CD43(-/-) T cells demonstrated no increase in tyrosine phosphorylation but a decreased calcium flux. Interestingly, CD43(-/-) T cells preferentially differentiated into Th2 cells in vitro, and CD43(-/-) T cells show increased GATA-3 translocation into the nucleus. In vivo, CD43(-/-) mice exhibited increased inflammation in two separate models of Th2-mediated allergic airway disease. In contrast, in Th1-mediated diabetes, nonobese diabetic CD43(-/-) mice did not significantly differ from wild-type mice in disease onset or progression. Th1-induced experimental autoimmune encephalomyelitis to MOG(35-55) was also normal in the CD43(-/-) mice. Nonetheless, the CD43(-/-) mice produced more IL-5 when restimulated with MOG(35-55) in vitro and demonstrated decreased delayed-type hypersensitivity responses. Together, these data demonstrate that although CD43(-/-) T cells preferentially differentiate into Th2 cells, this response is not sufficient to protect against Th1-mediated autoimmune responses.
Conflict of interest statement
Figures

References
-
- O'Garra A, Arai N. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol. 2000;10:542–550. - PubMed
-
- Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. - PubMed
-
- Boutin Y, Leitenberg D, Tao X, Bottomly K. Distinct biochemical signals characterize agonist- and altered peptide ligand-induced differentiation of naive CD4+ T cells into Th1 and Th2 subsets. J Immunol. 1997;159:5802–5809. - PubMed
-
- Rulifson IC, Sperling AI, Fields PE, Fitch FW, Bluestone JA. CD28 costimulation promotes the production of Th2 cytokines. J Immunol. 1997;158:658–665. - PubMed
-
- Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

