B lymphocyte stimulator regulates adaptive immune responses by directly promoting dendritic cell maturation
- PMID: 18490739
- PMCID: PMC2600490
- DOI: 10.4049/jimmunol.180.11.7394
B lymphocyte stimulator regulates adaptive immune responses by directly promoting dendritic cell maturation
Abstract
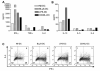
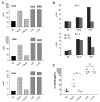
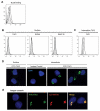
B lymphocyte stimulator (BLyS) is a well-known direct costimulator of adaptive immune cells, particularly B lineage cells. However, we have reported recently that BLyS is also able to activate monocytes. Other innate immune cells, such as dendritic cells (DCs), play a key role in the initiation of adaptive immune responses and the purpose of the current study was to assess whether there is a direct role for BLyS in modulating human DC functions. In this study, we show that BLyS induces DC activation and maturation. Thus, BLyS strongly induced up-regulation of surface costimulatory molecule expression and secretion of specific cytokines and chemokines in DCs. BLyS-stimulated DCs (BLyS-DCs) were also able to augment allogeneic CD4 T cell proliferation to a greater extent than control DCs. BLyS-DCs secreted elevated levels of the major Th1-polarizing cytokine, IL-12p70, and they promoted naive CD4 T cell differentiation into Th1 T cells. Regarding BLyS receptor expression, DCs primarily express cytoplasmic transmembrane activator and CAML interactor; however, low levels of cell surface transmembrane activator and CAML interactor are expressed as well. Collectively, our data suggest that BLyS may modulate adaptive immune cells indirectly by inducing DC maturation.
Figures







References
-
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. - PubMed
-
- Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–266. - PubMed
-
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. - PubMed
-
- Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205. - PubMed
-
- Chen M, Wang YH, Wang Y, Huang L, Sandoval H, Liu YJ, Wang J. Dendritic cell apoptosis in the maintenance of immune tolerance. Science. 2006;311:1160–1164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

