Requirement for enhancer specificity in immunoglobulin heavy chain locus regulation
- PMID: 18490744
- PMCID: PMC2408828
- DOI: 10.4049/jimmunol.180.11.7443
Requirement for enhancer specificity in immunoglobulin heavy chain locus regulation
Abstract
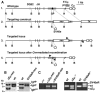
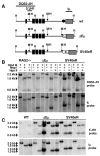
The intronic Emicro enhancer has been implicated in IgH locus transcription, VDJ recombination, class switch recombination, and somatic hypermutation. How Emicro controls these diverse mechanisms is still largely unclear, but transcriptional enhancer activity is thought to play a central role. In this study we compare the phenotype of mice lacking the Emicro element (DeltaEmicro) with that of mice in which Emu was replaced with the ubiquitous SV40 transcriptional enhancer (SV40eR mutation) and show that SV40e cannot functionally complement Emu loss in pro-B cells. Surprisingly, in fact, the SV40eR mutation yields a more profound defect than DeltaEmicro, with an almost complete block in micro0 germline transcription in pro-B cells. This active transcriptional suppression caused by enhancer replacement appears to be specific to the early stages of B cell development, as mature SV40eR B cells express micro0 transcripts at higher levels than DeltaEmicro mice and undergo complete DNA demethylation at the IgH locus. These results indicate an unexpectedly stringent, developmentally restricted requirement for enhancer specificity in regulating IgH function during the early phases of B cell differentiation, consistent with the view that coordination of multiple independent regulatory mechanisms and elements is essential for locus activation and VDJ recombination.
Figures



References
-
- Ernst P, Smale ST. Combinatorial regulation of transcription II: The immunoglobulin mu heavy chain gene. Immunity. 1995;2:427–438. - PubMed
-
- Bottaro A, Young F, Chen J, Serwe M, Sablitzky F, Alt FW. Deletion of the IgH intronic enhancer and associated matrix-attachment regions decreases, but does not abolish, class switching at the mu locus. Int Immunol. 1998;10:799–806. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

