Mast cell cathelicidin antimicrobial peptide prevents invasive group A Streptococcus infection of the skin
- PMID: 18490758
- PMCID: PMC2664112
- DOI: 10.4049/jimmunol.180.11.7565
Mast cell cathelicidin antimicrobial peptide prevents invasive group A Streptococcus infection of the skin
Abstract
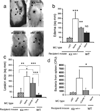
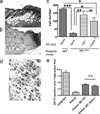
Mast cells (MC) express cathelicidin antimicrobial peptides that act as broad-spectrum antibiotics and influence the immune defense of multiple epithelial surfaces. We hypothesized that MC help protect against skin infection through the expression of cathelicidin. The susceptibility of MC-deficient mice (Kit Wsh(-/-)) to invasive group A streptococcus (GAS) was compared with control mice. Following s.c. injection of GAS, MC-deficient mice had 30% larger skin lesions, 80% more lesional bacteria, and 30% more spleens positive for bacteria. In contrast to results obtained when GAS was injected into skin, no significant differences were noted between MC-deficient mice and control mice after GAS was applied topically, indicating that MC activity is most important after barrier penetration. To determine whether these differences were due to MC expression of cathelicidin, MC-deficient mice were reconstituted with MC derived from either wild-type or cathelicidin-deficient (Camp(-/-)) mice and challenged with GAS. Forty-eight hours after bacterial injection, mice that did not receive MC had an average lesion size of 200 mm(2), mice reconstituted with wild-type MC showed lesions comparable to control mice (25 mm(2)), while mice reconstituted with Camp(-/-) MC showed an average lesion size of 120 mm(2). Surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF-MS) analysis of cathelicidin peptide purified from mast cells defined this as a unique 28-aa peptide. Combined, these results show that MC confer defense against Gram-positive bacterial infection in the skin, a function mediated in part by the expression of a unique cathelicidin peptide.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures




References
-
- Malaviya R, Ikeda T, Ross EA, Jakschik BA, Abraham SN. Bacteria: mast cell interactions in inflammatory disease. Am. J. Ther. 1995;2:787–792. - PubMed
-
- Malaviya R, Abraham SN. Mast cell modulation of immune responses to bacteria. Immunol. Rev. 2001;179:16–24. - PubMed
-
- Di Nardo A, Vitiello A, Gallo RL. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J. Immunol. 2003;170:2274–2278. - PubMed
-
- Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

