Roles of cyclins A and E in induction of centrosome amplification in p53-compromised cells
- PMID: 18490919
- PMCID: PMC2574884
- DOI: 10.1038/onc.2008.161
Roles of cyclins A and E in induction of centrosome amplification in p53-compromised cells
Abstract
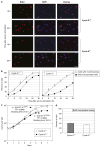
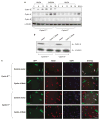
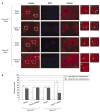
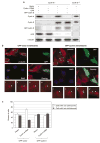
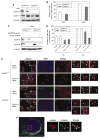
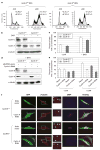
Abnormal amplification of centrosomes, which occurs frequently in cancers, leads to high frequencies of mitotic defect and chromosome segregation error, profoundly affecting the rate of tumor progression. Centrosome amplification results primarily from overduplication of centrosomes, and p53 is involved in the regulation of centrosome duplication partly through controlling the activity of cyclin-dependent kinase (CDK) 2-cyclin E, a kinase complex critical for the initiation of centrosome duplication. Thus, loss or mutational inactivation of p53 leads to an increased frequency of centrosome amplification. Moreover, the status of cyclin E greatly influences the frequency of centrosome amplification in cells lacking functional p53. Here, we dissected the roles of CDK2-associating cyclins, namely cyclins E and A, in centrosome amplification in the p53-negative cells. We found that loss of cyclin E was readily compensated by cyclin A for triggering the initiation of centrosome duplication, and thus the centrosome duplication kinetics was not significantly altered in cyclin E-deficient cells. It has been shown that cells lacking functional p53, when arrested in either early S-phase or late G(2) phase, continue to reduplicate centrosomes, resulting in centrosome amplification. In cells arrested in early S phase, cyclin E, but not cyclin A, is important in centrosome amplification, whereas in the absence of cyclin E, cyclin A is important for centrosome amplification. In late G(2)-arrested cells, cyclin A is important in centrosome amplification irrespective of the cyclin E status. These findings advance our understandings of the mechanisms underlying the numeral abnormality of centrosomes and consequential genomic instability associated with loss of p53 function and aberrant expression of cyclins E and A in cancer cells.
Figures


References
-
- Bennett RA, Izumi H, Fukasawa K. Induction of centrosome amplification and chromosome instability in p53-null cells by transient exposure to subtoxic levels of S-phase-targeting anticancer drugs. Oncogene. 2004;23:6823–6829. - PubMed
-
- Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. - PubMed
-
- Carroll PE, Okuda M, Horn HF, Biddinger P, Stambrook PJ, Gleich LL, et al. Centrosome hyperamplification in human cancer: chromosome instability induced by p53 mutation and/or Mdm2 overexpression. Oncogene. 1999;18:1935–1944. - PubMed
-
- Coverley D, Laman H, Laskey RA. Distinct roles for cyclins E and A during DNA replication complex assembly and activation. Nat Cell Biol. 2002;4:523–528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

