Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera
- PMID: 18490996
- PMCID: PMC2104745
- DOI: 10.3114/sim.2007.58.03
Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera
Abstract
The phylogeny of the genera Periconiella, Ramichloridium, Rhinocladiella and Veronaea was explored by means of partial sequences of the 28S (LSU) rRNA gene and the ITS region (ITS1, 5.8S rDNA and ITS2). Based on the LSU sequence data, ramichloridium-like species segregate into eight distinct clusters. These include the Capnodiales (Mycosphaerellaceae and Teratosphaeriaceae), the Chaetothyriales (Herpotrichiellaceae), the Pleosporales, and five ascomycete clades with uncertain affinities. The type species of Ramichloridium, R. apiculatum, together with R. musae, R. biverticillatum, R. cerophilum, R. verrucosum, R. pini, and three new species isolated from Strelitzia, Musa and forest soil, respectively, reside in the Capnodiales clade. The human-pathogenic species R. mackenziei and R. basitonum, together with R. fasciculatum and R. anceps, cluster with Rhinocladiella (type species: Rh. atrovirens, Herpotrichiellaceae, Chaetothyriales), and are allocated to this genus. Veronaea botryosa, the type species of the genus Veronaea, also resides in the Chaetothyriales clade, whereas Veronaea simplex clusters as a sister taxon to the Venturiaceae (Pleosporales), and is placed in a new genus, Veronaeopsis. Ramichloridium obovoideum clusters with Carpoligna pleurothecii (anamorph: Pleurothecium sp., Chaetosphaeriales), and a new combination is proposed in Pleurothecium. Other ramichloridium-like clades include R. subulatum and R. epichloës (incertae sedis, Sordariomycetes), for which a new genus, Radulidium is erected. Ramichloridium schulzeri and its varieties are placed in a new genus, Myrmecridium (incertae sedis, Sordariomycetes). The genus Pseudovirgaria (incertae sedis) is introduced to accommodate ramichloridium-like isolates occurring on various species of rust fungi. A veronaea-like isolate from Bertia moriformis with phylogenetic affinity to the Annulatascaceae (Sordariomycetidae) is placed in a new genus, Rhodoveronaea. Besides Ramichloridium, Periconiella is also polyphyletic. Thysanorea is introduced to accommodate Periconiella papuana (Herpotrichiellaceae), which is unrelated to the type species, P. velutina (Mycosphaerellaceae).
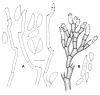
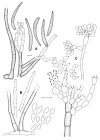
Figures




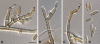









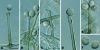

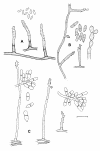
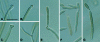









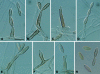



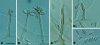


References
-
- Al-Hedaithy SSA, Jamjoom ZAB, Saeed ES (1988). Cerebral phaeohyphomycosis caused by Fonsecaea pedrosoi in Saudi-Arabia. Acta Pathologica Microbiologica Scandinaviae 96Suppl. 3: 94-100. - PubMed
-
- Aptroot A (2006). Mycosphaerella and its anamorphs: 2. Conspectus of Mycosphaerella. CBS Biodiversity Series 5: 1-231.
-
- Braun U (1998). A monograph of Cercosporella, Ramularia and allied genera (phytopathogenic hypomycetes). Vol. 2. IHW-Verlag, Eching.
-
- Braun U, Crous PW, Dugan F, Groenewald JZ, Hoog GS de (2003). Phylogeny and taxonomy of cladosporium-like hyphomycetes, including Davidiella gen. nov., the teleomorph of Cladosporium s. str. Mycological Progress 2: 3-18.
-
- Campbell CK, Al-Hedaithy SSA (1993). Phaeohyphomycosis of the brain caused by Ramichloridium mackenziei sp. nov. in middle eastern countries. Journal of Medical and Veterinary Mycology 31: 325-332.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
