Linear ensemble-coding in midbrain superior colliculus specifies the saccade kinematics
- PMID: 18491166
- PMCID: PMC2798131
- DOI: 10.1007/s00422-008-0219-z
Linear ensemble-coding in midbrain superior colliculus specifies the saccade kinematics
Abstract
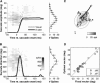
Recently, we proposed an ensemble-coding scheme of the midbrain superior colliculus (SC) in which, during a saccade, each spike emitted by each recruited SC neuron contributes a fixed minivector to the gaze-control motor output. The size and direction of this 'spike vector' depend exclusively on a cell's location within the SC motor map (Goossens and Van Opstal, in J Neurophysiol 95: 2326-2341, 2006). According to this simple scheme, the planned saccade trajectory results from instantaneous linear summation of all spike vectors across the motor map. In our simulations with this model, the brainstem saccade generator was simplified by a linear feedback system, rendering the total model (which has only three free parameters) essentially linear. Interestingly, when this scheme was applied to actually recorded spike trains from 139 saccade-related SC neurons, measured during thousands of eye movements to single visual targets, straight saccades resulted with the correct velocity profiles and nonlinear kinematic relations ('main sequence properties' and 'component stretching'). Hence, we concluded that the kinematic nonlinearity of saccades resides in the spatial-temporal distribution of SC activity, rather than in the brainstem burst generator. The latter is generally assumed in models of the saccadic system. Here we analyze how this behaviour might emerge from this simple scheme. In addition, we will show new experimental evidence in support of the proposed mechanism.
Figures






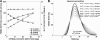
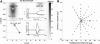



Similar articles
-
Optimal control of saccades by spatial-temporal activity patterns in the monkey superior colliculus.PLoS Comput Biol. 2012;8(5):e1002508. doi: 10.1371/journal.pcbi.1002508. Epub 2012 May 17. PLoS Comput Biol. 2012. PMID: 22615548 Free PMC article.
-
Dynamic ensemble coding of saccades in the monkey superior colliculus.J Neurophysiol. 2006 Apr;95(4):2326-41. doi: 10.1152/jn.00889.2005. Epub 2005 Dec 21. J Neurophysiol. 2006. PMID: 16371452
-
Activity of neurons in monkey superior colliculus during interrupted saccades.J Neurophysiol. 1996 Jun;75(6):2562-80. doi: 10.1152/jn.1996.75.6.2562. J Neurophysiol. 1996. PMID: 8793764
-
Linking express saccade occurance to stimulus properties and sensorimotor integration in the superior colliculus.J Neurophysiol. 2015 Aug;114(2):879-92. doi: 10.1152/jn.00047.2015. Epub 2015 Jun 10. J Neurophysiol. 2015. PMID: 26063770 Free PMC article.
-
Microstimulation in a spiking neural network model of the midbrain superior colliculus.PLoS Comput Biol. 2019 Apr 12;15(4):e1006522. doi: 10.1371/journal.pcbi.1006522. eCollection 2019 Apr. PLoS Comput Biol. 2019. PMID: 30978180 Free PMC article.
Cited by
-
Error inconsistency does not generally inhibit saccadic adaptation: Support for linear models of multi-gainfield adaptation.Physiol Rep. 2022 Feb;10(4):e15180. doi: 10.14814/phy2.15180. Physiol Rep. 2022. PMID: 35212174 Free PMC article.
-
Neural mechanisms of oculomotor abnormalities in the infantile strabismus syndrome.J Neurophysiol. 2017 Jul 1;118(1):280-299. doi: 10.1152/jn.00934.2016. Epub 2017 Apr 12. J Neurophysiol. 2017. PMID: 28404829 Free PMC article. Review.
-
Ventral premotor cortex encodes task relevant features during eye and head movements.Sci Rep. 2022 Dec 21;12(1):22093. doi: 10.1038/s41598-022-26479-2. Sci Rep. 2022. PMID: 36543870 Free PMC article.
-
Motor functions of the superior colliculus.Annu Rev Neurosci. 2011;34:205-31. doi: 10.1146/annurev-neuro-061010-113728. Annu Rev Neurosci. 2011. PMID: 21456962 Free PMC article. Review.
-
Experimental tests of hypotheses for microsaccade generation.Exp Brain Res. 2015 Apr;233(4):1089-95. doi: 10.1007/s00221-014-4188-2. Epub 2015 Jan 7. Exp Brain Res. 2015. PMID: 25563497
References
-
- Bahill AT, Clark MR, Stark L. The main sequence, a tool for studying human eye movements. Math Biosci. 1977;24:191–204. doi: 10.1016/0025-5564(75)90075-9. - DOI
-
- Crawford JD, Guitton D. Visual-motor transformations required for accurate and kinematically correct saccades. J Neurophysiol. 1997;78:1447–1467. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

