Inhibition of ERK promotes collagen gel compaction and fibrillogenesis to amplify the osteogenesis of human mesenchymal stem cells in three-dimensional collagen I culture
- PMID: 18491946
- PMCID: PMC2656582
- DOI: 10.1089/scd.2008.0075
Inhibition of ERK promotes collagen gel compaction and fibrillogenesis to amplify the osteogenesis of human mesenchymal stem cells in three-dimensional collagen I culture
Abstract
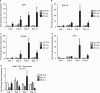
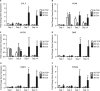
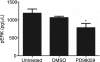
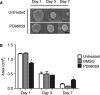
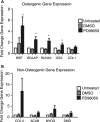
Tissue morphogenesis remains one of the least understood problems in cell and developmental biology. There is a disconnect between the mechanisms that apply to two-dimensional (2D) cultures and those seen in vivo. Three-dimensional (3D) culture presents a complex stimulus triggering cellular responses that are only partially understood. We compared 2D and 3D cultures of human mesenchymal stem cells in the presence of mitogen-activated protein kinase kinase (MEK) inhibitor, PD98059, to determine the role of extracellular signal-related kinase (ERK) in collagen-induced differentiation. 3D collagen I culture enhanced and accelerated the osteogenic differentiation of human mesenchymal stem cells (hMSC). Contrary to 2D results, the addition of PD98059 induced a significant amplification of osteogenic gene expression and matrix mineralization in 3D cultures. The inhibition of ERK altered cell-mediated compaction, proliferation, and resulted in the development of distinct tissue microstructure. Therefore, we suggest that the ability to reorganize collagen in 3D is an important step in ERK-mediated osteogenic differentiation. This work aims to propose a correlation between osteogenic differentiation and hMSC-directed collagen I remodeling. We present a potential mechanistic link (ERK) through which the three dimensionality of an engineered tissue acts to differentially induce and maintain cellular phenotype during tissue development.
Figures
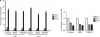


Similar articles
-
A novel collagen-binding peptide promotes osteogenic differentiation via Ca2+/calmodulin-dependent protein kinase II/ERK/AP-1 signaling pathway in human bone marrow-derived mesenchymal stem cells.Cell Signal. 2008 Apr;20(4):613-24. doi: 10.1016/j.cellsig.2007.11.012. Epub 2007 Nov 29. Cell Signal. 2008. PMID: 18248957
-
Icariin induces osteogenic differentiation of bone mesenchymal stem cells in a MAPK-dependent manner.Cell Prolif. 2015 Jun;48(3):375-84. doi: 10.1111/cpr.12185. Epub 2015 Apr 13. Cell Prolif. 2015. PMID: 25867119 Free PMC article.
-
Extracellular-signal-related kinase 1/2 is responsible for inhibition of osteogenesis in three-dimensional cultured MC3T3-E1 cells.Tissue Eng Part A. 2010 Nov;16(11):3485-94. doi: 10.1089/ten.TEA.2010.0222. Epub 2010 Sep 9. Tissue Eng Part A. 2010. PMID: 20590408
-
Gene expression profile of human mesenchymal stem cells during osteogenesis in three-dimensional thermoreversible gelation polymer.Biochem Biophys Res Commun. 2004 May 14;317(4):1103-7. doi: 10.1016/j.bbrc.2004.03.165. Biochem Biophys Res Commun. 2004. PMID: 15094382
-
Effects of micropitted/nanotubular titania topographies on bone mesenchymal stem cell osteogenic differentiation.Biomaterials. 2012 Mar;33(9):2629-41. doi: 10.1016/j.biomaterials.2011.12.024. Epub 2011 Dec 26. Biomaterials. 2012. PMID: 22204980
Cited by
-
The role of hesperetin on osteogenesis of human mesenchymal stem cells and its function in bone regeneration.Oncotarget. 2017 Mar 28;8(13):21031-21043. doi: 10.18632/oncotarget.15473. Oncotarget. 2017. PMID: 28423500 Free PMC article.
-
Extracellular matrix protein adsorption to phosphate-functionalized gels from serum promotes osteogenic differentiation of human mesenchymal stem cells.Acta Biomater. 2013 Jan;9(1):4525-34. doi: 10.1016/j.actbio.2012.09.007. Epub 2012 Sep 13. Acta Biomater. 2013. PMID: 22982322 Free PMC article.
-
Bone repair and key signalling pathways for cell-based bone regenerative therapy: A review.J Taibah Univ Med Sci. 2023 May 24;18(6):1350-1363. doi: 10.1016/j.jtumed.2023.05.015. eCollection 2023 Dec. J Taibah Univ Med Sci. 2023. PMID: 37305024 Free PMC article. Review.
-
Effects of matrix metalloproteinases on the fate of mesenchymal stem cells.Stem Cell Res Ther. 2016 Sep 9;7(1):129. doi: 10.1186/s13287-016-0393-1. Stem Cell Res Ther. 2016. PMID: 27612636 Free PMC article. Review.
-
Osteogenic cells form mineralized particles, a few μm in size, in a 3D collagen gel culture.PeerJ. 2019 Oct 23;7:e7889. doi: 10.7717/peerj.7889. eCollection 2019. PeerJ. 2019. PMID: 31660270 Free PMC article.
References
-
- Jaiswal RK. Jaiswal N. Bruder SP. Mbalaviele G. Marshak DR. Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275:9645–9652. - PubMed
-
- Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD. Moorman MA. Simonetti DW. Craig S. Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
-
- Jaiswal N. Haynesworth SE. Caplan AI. Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. - PubMed
-
- Haynesworth SE. Goshima J. Goldberg VM. Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

