Valganciclovir for suppression of human herpesvirus-8 replication: a randomized, double-blind, placebo-controlled, crossover trial
- PMID: 18491970
- PMCID: PMC2700177
- DOI: 10.1086/588820
Valganciclovir for suppression of human herpesvirus-8 replication: a randomized, double-blind, placebo-controlled, crossover trial
Abstract
Background: Human herpesvirus-8 (HHV-8) replication is critical in the induction and maintenance of Kaposi sarcoma, primary effusion lymphoma, and some cases of Castleman disease. In vitro and observational studies suggest that ganciclovir inhibits HHV-8 replication, but no randomized clinical trials have been conducted.
Methods: A total of 26 men infected with HHV-8 were randomized to receive 8 weeks of valganciclovir administered orally (900 mg once per day) or 8 weeks of placebo administered orally. After a 2-week washout period, participants in each group received the study drug they had not yet taken (either valganciclovir or placebo), for 8 additional weeks. Oral swab samples were collected daily during the study, and HHV-8 and CMV DNA were quantified by real-time PCR.
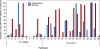
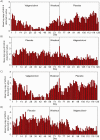
Results: A total of 16 human immunodeficiency virus (HIV)-positive men and 10 HIV-negative men enrolled in and completed the study. Of the 3,439 swab samples that participants had been expected to provide, 3029 (88%) were available for analysis. HHV-8 was detected on 44% of swabs collected from participants who were receiving placebo, compared with 23% of swabs collected from participants who were receiving valganciclovir (relative risk [RR], 0.54 [95% confidence interval {CI}, 0.33-0.90]; P = .02). Valganciclovir reduced oropharyngeal shedding of cytomegalovirus by 80% (RR, 0.20 [95% CI, 0.08-0.48]; P < .001). Shedding of HHV-8 and shedding of cytomegalovirus were independent. Hematologic, renal, or hepatic toxicities were no more common among participants who received the active drug, compared with those who received placebo, though participants who received valganciclovir reported more days of diarrhea.
Conclusions: Valganciclovir administered orally once per day is well tolerated and significantly reduces the frequency and quantity of HHV-8 replication.
Figures

Comment in
-
Valganciclovir and human herpesvirus-8.J Infect Dis. 2008 Jul 1;198(1):6-7. doi: 10.1086/588821. J Infect Dis. 2008. PMID: 18491975 No abstract available.
References
-
- Antman K, Chang Y. Kaposi's sarcoma. N Engl J Med. 2000;342:1027–38. - PubMed
-
- Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst. 2000;92:1823–30. - PubMed
-
- Eltom MA, Jemal A, Mbulaiteye SM, Devesa SS, Biggar RJ. Trends in Kaposi's sarcoma and non-Hodgkin's lymphoma incidence in the United States from 1973 through 1998. J Natl Cancer Inst. 2002;94:1204–10. - PubMed
-
- Qunibi W, Al-Furayh O, Almeshari K, et al. Serologic association of human herpesvirus eight with posttransplant Kaposi's sarcoma in Saudi Arabia. Transplantation. 1998;65:583–5. - PubMed
-
- Cancer incidence in five continents. IARC Sci Publ. 2002;VIII:1–781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

