Curcumin reduces the toxic effects of iron loading in rat liver epithelial cells
- PMID: 18492020
- PMCID: PMC2614453
- DOI: 10.1111/j.1478-3231.2008.01793.x
Curcumin reduces the toxic effects of iron loading in rat liver epithelial cells
Abstract
Background/aims: Iron overload can cause liver toxicity and increase the risk of liver failure or hepatocellular carcinoma in humans. Curcumin (diferuloylmethane), a component of the food spice turmeric, has antioxidant, iron binding and hepatoprotective properties. The aim of this study was to quantify its effects on iron overload and the resulting downstream toxic effects in cultured T51B rat liver epithelial cells.
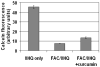
Methods: T51B cells were loaded with ferric ammonium citrate (FAC) with or without the iron delivery agent 8-hydroxyquinoline. Cytotoxicity was measured by methylthiazolyldiphenyl-tetrazolium bromide assay. Iron uptake and iron bioavailability were documented by chemical assay, quench of calcein fluorescence and ferritin induction. Reactive oxygen species (ROS) were measured by a fluorescence assay using 2',7'-dichlorodihydrofluorescein diacetate. Oxidative stress signalling to jnk, c-jun and p38 was measured by a Western blot with phospho-specific antibodies.
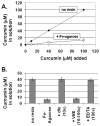
Results: Curcumin bound iron, but did not block iron uptake or bioavailability in T51B cells given FAC. However, it reduced cytotoxicity, blocked the generation of ROS and eliminated signalling to cellular stress pathways caused by iron. Inhibition was observed over a wide range of FAC concentrations (50-500 microM), with an apparent IC(50) in all cases between 5 and 10 microM curcumin. In contrast, desferoxamine blocked both iron uptake and toxic effects of iron at concentrations that depended on the FAC concentration. The effects of curcumin also differed from those of alpha-tocopherol, which did not bind iron and was less effective at blocking iron-stimulated ROS generation.
Conclusions: Curcumin reduced iron-dependent oxidative stress and iron toxicity in T51B cells without blocking iron uptake.
Figures








Similar articles
-
Neoplastic transformation of rat liver epithelial cells is enhanced by non-transferrin-bound iron.BMC Gastroenterol. 2008 Feb 6;8:2. doi: 10.1186/1471-230X-8-2. BMC Gastroenterol. 2008. PMID: 18254965 Free PMC article.
-
Curcumin and Turmeric Modulate the Tumor-Promoting Effects of Iron In Vitro.Nutr Cancer. 2017 Apr;69(3):481-489. doi: 10.1080/01635581.2017.1274407. Epub 2017 Jan 27. Nutr Cancer. 2017. PMID: 28129008 Free PMC article.
-
Isolation and characterization of iron chelators from turmeric (Curcuma longa): selective metal binding by curcuminoids.Biometals. 2017 Oct;30(5):699-708. doi: 10.1007/s10534-017-0038-6. Epub 2017 Aug 11. Biometals. 2017. PMID: 28801864 Free PMC article.
-
Iron overload induced by ferric ammonium citrate triggers reactive oxygen species-mediated apoptosis via both extrinsic and intrinsic pathways in human hepatic cells.Hum Exp Toxicol. 2016 Jun;35(6):598-607. doi: 10.1177/0960327115597312. Epub 2015 Jul 29. Hum Exp Toxicol. 2016. PMID: 26224043
-
Iron overload causes oxidative stress and impaired insulin signaling in AML-12 hepatocytes.Dig Dis Sci. 2013 Jul;58(7):1899-908. doi: 10.1007/s10620-013-2648-3. Epub 2013 Apr 5. Dig Dis Sci. 2013. PMID: 23558563 Free PMC article.
Cited by
-
Iron chelation by curcumin suppresses both curcumin-induced autophagy and cell death together with iron overload neoplastic transformation.Cell Death Discov. 2019 Dec 9;5:150. doi: 10.1038/s41420-019-0234-y. eCollection 2019. Cell Death Discov. 2019. PMID: 31839992 Free PMC article.
-
Towards a unifying, systems biology understanding of large-scale cellular death and destruction caused by poorly liganded iron: Parkinson's, Huntington's, Alzheimer's, prions, bactericides, chemical toxicology and others as examples.Arch Toxicol. 2010 Nov;84(11):825-89. doi: 10.1007/s00204-010-0577-x. Epub 2010 Aug 17. Arch Toxicol. 2010. PMID: 20967426 Free PMC article. Review.
-
Curcumin Nanocarriers in the Protection Against Iron- and Alcohol-Induced Oxidative Stress in a Cellular Model of Liver Disease.Biology (Basel). 2025 Apr 23;14(5):455. doi: 10.3390/biology14050455. Biology (Basel). 2025. PMID: 40427645 Free PMC article.
-
Curcumin inhibits activation of TRPM2 channels in rat hepatocytes.Redox Biol. 2016 Apr;7:1-7. doi: 10.1016/j.redox.2015.11.001. Epub 2015 Nov 10. Redox Biol. 2016. PMID: 26609559 Free PMC article.
-
Alcoholic fatty liver disease inhibited the co-expression of Fmo5 and PPARα to activate the NF-κB signaling pathway, thereby reducing liver injury via inducing gut microbiota disturbance.J Exp Clin Cancer Res. 2021 Jan 7;40(1):18. doi: 10.1186/s13046-020-01782-w. J Exp Clin Cancer Res. 2021. PMID: 33413501 Free PMC article.
References
-
- Stal P. Iron as a hepatotoxin. Dig Dis. 1995;13(4):205–222. - PubMed
-
- Eaton JW, Qian M. Molecular bases of cellular iron toxicity. Free Radic Biol Med. 2002;32(9):833–840. - PubMed
-
- Haber F, Weiss J. The catalytic decompostion of hydrogen peroxide by iron salts. Proc Royal Society London Section A. 1934;147:332–351.
-
- Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12(10):1161–1208. - PubMed
-
- Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

