Functional roles of N-glycans in cell signaling and cell adhesion in cancer
- PMID: 18492092
- PMCID: PMC11158068
- DOI: 10.1111/j.1349-7006.2008.00839.x
Functional roles of N-glycans in cell signaling and cell adhesion in cancer
Abstract
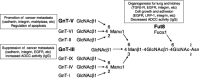
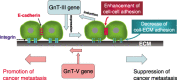
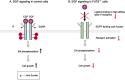
Glycosylation is one of the most common post-translational modification reactions and nearly half of all known proteins in eukaryotes are glycosylated. In fact, changes in oligosaccharide structures are associated with many physiological and pathological events, including cell growth, migration, differentiation, tumor invasion, host-pathogen interactions, cell trafficking, and transmembrane signaling. Emerging roles of glycan functions have been highly attractive to scientists in various fields of life science as they open a field, "Functional Glycomics", that is a comprehensive study of the glycan structures in relation to functions. In particular, the N-glycans of signaling molecules including receptors or adhesion molecules are considered to be involved in cellular functions. This review will focus on the roles of glycosyltransferases involved in the biosynthesis of N-glycan branching and identification of cell surface receptors as their target proteins. We also suggest that the modulation of N-glycans of those receptors alters their important functions such as cell signaling and cell adhesion which are implicated in cancer invasion and metastasis.
Figures
Similar articles
-
Core fucose and bisecting GlcNAc, the direct modifiers of the N-glycan core: their functions and target proteins.Carbohydr Res. 2009 Aug 17;344(12):1387-90. doi: 10.1016/j.carres.2009.04.031. Epub 2009 May 4. Carbohydr Res. 2009. PMID: 19508951 Review.
-
[N-glycans-based regulation of integrin-mediated cell adhesion].Tanpakushitsu Kakusan Koso. 2008 Sep;53(12 Suppl):1508-12. Tanpakushitsu Kakusan Koso. 2008. PMID: 21089357 Review. Japanese. No abstract available.
-
Importance of N-glycosylation on alpha5beta1 integrin for its biological functions.Biol Pharm Bull. 2009 May;32(5):780-5. doi: 10.1248/bpb.32.780. Biol Pharm Bull. 2009. PMID: 19420742 Review.
-
Potential of N-glycan in cell adhesion and migration as either a positive or negative regulator.Cell Adh Migr. 2008 Oct-Dec;2(4):243-5. doi: 10.4161/cam.2.4.6748. Epub 2008 Oct 5. Cell Adh Migr. 2008. PMID: 19262156 Free PMC article.
-
[Regulation of integrin functions by N-glycans].Yakugaku Zasshi. 2007 Apr;127(4):571-8. doi: 10.1248/yakushi.127.571. Yakugaku Zasshi. 2007. PMID: 17409685 Review. Japanese.
Cited by
-
O-mannosylation and N-glycosylation: two coordinated mechanisms regulating the tumour suppressor functions of E-cadherin in cancer.Oncotarget. 2016 Oct 4;7(40):65231-65246. doi: 10.18632/oncotarget.11245. Oncotarget. 2016. PMID: 27533452 Free PMC article.
-
Comparison of self-sampling blood collection for N-glycosylation analysis.BMC Res Notes. 2022 Feb 16;15(1):61. doi: 10.1186/s13104-022-05958-9. BMC Res Notes. 2022. PMID: 35172879 Free PMC article.
-
Systematic synthesis of bisected N-glycans and unique recognitions by glycan-binding proteins.Chem Sci. 2022 Jun 9;13(25):7644-7656. doi: 10.1039/d1sc05435j. eCollection 2022 Jun 29. Chem Sci. 2022. PMID: 35872821 Free PMC article.
-
A haploid genetic screen identifies the major facilitator domain containing 2A (MFSD2A) transporter as a key mediator in the response to tunicamycin.Proc Natl Acad Sci U S A. 2011 Jul 19;108(29):11756-65. doi: 10.1073/pnas.1018098108. Epub 2011 Jun 15. Proc Natl Acad Sci U S A. 2011. PMID: 21677192 Free PMC article.
-
Altered Glycosylation in Progression and Management of Bladder Cancer.Molecules. 2023 Apr 13;28(8):3436. doi: 10.3390/molecules28083436. Molecules. 2023. PMID: 37110670 Free PMC article. Review.
References
-
- Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS‐PROT database. Biochim Biophys Acta 1999; 1473: 4–8. - PubMed
-
- Taniguchi N, Honke K, Fukuda M, eds. Handbook of Glycosyltransferases and Related Genes. Tokyo: Springer, 2002.
-
- Narimatsu H. Human glycogene cloning: focus on beta 3‐glycosyltransferase and beta 4‐glycosyltransferase families. Curr Opin Struct Biol 2006; 16: 567–75. - PubMed
-
- Dwek RA. Glycobiology. ‘towards understanding the function of sugars’. Biochem Soc Trans 1995; 23: 1–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

