18-month occurrence of severe events among early diagnosed HIV-infected children before antiretroviral therapy in Abidjan, Côte d'Ivoire: a cohort study
- PMID: 18492259
- PMCID: PMC2416449
- DOI: 10.1186/1471-2458-8-169
18-month occurrence of severe events among early diagnosed HIV-infected children before antiretroviral therapy in Abidjan, Côte d'Ivoire: a cohort study
Abstract
Objective: To assess the 18-month field effectiveness on severe events of a pediatric package combining early HIV-diagnosis and targeted cotrimoxazole prophylaxis in HIV-infected children from age six-week before the antiretroviral era, in Abidjan, Côte d'Ivoire.
Methods: Data from two consecutive prevention of HIV mother-to-child transmission programs were compared: the ANRS 1201/1202 Ditrame-Plus cohort (2001-2005) and the pooled data of the ANRS 049a Ditrame randomized trial and its following open-labeled cohort (1995-2000), used as a reference group. HIV-infected pregnant women > or = 32-36 weeks of gestation were offered a short-course peri-partum antiretroviral prophylaxis (ZDV in Ditrame, and ZDV +/- 3TC+single-dose (sd) NVP in Ditrame-Plus). Neonatal prophylaxis was provided in Ditrame-Plus only: 7-day ZDV and sdNVP 48-72 h after birth. A 6-week pediatric HIV-RNA diagnosis was provided on-line in the Ditrame-Plus while it was only oriented on clinical symptoms in Ditrame. Six-week HIV-infected children received a daily cotrimoxazole prophylaxis in Ditrame-Plus while no prophylaxis was provided in Ditrame. The determinants of severe events (death or hospitalization > 1 day) were assessed in a Cox regression model.
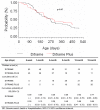
Results: Between 1995 and 2003, 98 out of the 1121 live-births were diagnosed as HIV-infected in peri-partum: 45 from Ditrame-Plus and 53 from Ditrame. The 18-month Kaplan-Meier cumulative probability of presenting a severe event was 66% in Ditrame-Plus (95% confidence interval [95%CI]: 50%-81%) and 77% in Ditrame (95%CI: 65%-89%), Log Rank test: p = 0.47. After adjustment on maternal WHO clinical stage, maternal death, 6-week pediatric viral load, birth-weight, and breastfeeding exposure, the 18-month risk of severe event was lower in Ditrame-Plus than in Ditrame (adjusted Hazard Ratio (aHR): 0.55, 95%CI: 0.3-1.1), although the difference was not statistically significant; p = 0.07). Maternal death was the only variable determinant of the occurrence of severe events in children (aHR: 3.73; CI: 2.2-11.2; p = 0.01).
Conclusion: Early cotrimoxazole from 6 weeks of age in HIV-infected infants seemed to reduce probability of severe events but the study lacked statistical power to prove this. Even with systematic cotrimoxazole prophylaxis, infant morbidity and mortality remained high pointing towards a need for early pediatric HIV-diagnosis and antiretroviral treatment in Africa.
Figures
Similar articles
-
18-month effectiveness of short-course antiretroviral regimens combined with alternatives to breastfeeding to prevent HIV mother-to-child transmission.PLoS One. 2008 Feb 20;3(2):e1645. doi: 10.1371/journal.pone.0001645. PLoS One. 2008. PMID: 18286200 Free PMC article.
-
Two-year morbidity-mortality and alternatives to prolonged breast-feeding among children born to HIV-infected mothers in Côte d'Ivoire.PLoS Med. 2007 Jan;4(1):e17. doi: 10.1371/journal.pmed.0040017. PLoS Med. 2007. PMID: 17227132 Free PMC article. Clinical Trial.
-
Early mixed feeding and breastfeeding beyond 6 months increase the risk of postnatal HIV transmission: ANRS 1201/1202 Ditrame Plus, Abidjan, Côte d'Ivoire.Prev Med. 2008 Jul;47(1):27-33. doi: 10.1016/j.ypmed.2007.11.014. Epub 2007 Dec 4. Prev Med. 2008. PMID: 18190955
-
Cotrimoxazole prophylaxis in adults infected with HIV in low-income countries.Curr Opin Infect Dis. 2001 Oct;14(5):507-12. doi: 10.1097/00001432-200110000-00002. Curr Opin Infect Dis. 2001. PMID: 11964869 Review.
-
Estimating the impact of alternative programmatic cotrimoxazole strategies on mortality among children born to mothers with HIV: A modelling study.PLoS Med. 2024 Feb 20;21(2):e1004334. doi: 10.1371/journal.pmed.1004334. eCollection 2024 Feb. PLoS Med. 2024. PMID: 38377150 Free PMC article. Review.
Cited by
-
Obstetrical, maternal characteristics and outcome of HIV-infected rapid progressor infants at Yaounde: a retrospective study.Transl Pediatr. 2016 Apr;5(2):46-54. doi: 10.21037/tp.2016.04.04. Transl Pediatr. 2016. PMID: 27186521 Free PMC article.
-
Association between age at antiretroviral therapy initiation and 24-month immune response in West-African HIV-infected children.AIDS. 2014 Jul 17;28(11):1645-55. doi: 10.1097/QAD.0000000000000272. AIDS. 2014. PMID: 24804858 Free PMC article.
-
Severe morbidity and mortality in untreated HIV-infected children in a paediatric care programme in Abidjan, Côte d'Ivoire, 2004-2009.BMC Infect Dis. 2011 Jun 23;11:182. doi: 10.1186/1471-2334-11-182. BMC Infect Dis. 2011. PMID: 21699728 Free PMC article.
-
Health care resource utilization in untreated HIV-infected children in a pediatric programme, Abidjan, Côte d'Ivoire, 2004-2009.J Acquir Immune Defic Syndr. 2013 Jan 1;62(1):e14-21. doi: 10.1097/QAI.0b013e3182739c95. J Acquir Immune Defic Syndr. 2013. PMID: 23262977 Free PMC article.
-
Incidence of World Health Organization stage 3 and 4 events, tuberculosis and mortality in untreated, HIV-infected children enrolling in care before 1 year of age: an IeDEA (International Epidemiologic Databases To Evaluate AIDS) East Africa regional analysis.Pediatr Infect Dis J. 2014 Jun;33(6):623-9. doi: 10.1097/INF.0000000000000223. Pediatr Infect Dis J. 2014. PMID: 24378935 Free PMC article.
References
-
- World Health Organisation . Antiretroviral drugs and the prevention of mother-to-child transmission in resource-limited settings. Recommendations for a public health approach (2005 revision). Technical Reference Group, Geneva, Switzerland, 28-29th June 2005. Geneva , WHO; 2005. p. 4.
-
- Leroy V, Sakarovitch C, Cortina-Borja M, McIntyre J, Coovadia H, Dabis F, Newell ML, Saba J, Gray G, Ndugwa C, Kilewo C, Massawe A, Kituuka P, Okong P, Grulich A, von Briesen H, Goudsmit J, Biberfeld G, Haverkamp G, Weverling GJ, Lange JM. Is there a difference in the efficacy of peripartum antiretroviral regimens in reducing mother-to-child transmission of HIV in Africa? AIDS. 2005;19:1865–1875. doi: 10.1097/01.aids.0000188423.02786.55. - DOI - PubMed
-
- World Health Organisation. UNAIDS. UNICEF . Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Progress report, April 2007. Geneve , World Health Organisation; 2007. p. 82.
-
- AIDS Epidemic Update. http://www.unaids.org/en/HIV_data/2006GlobalReport/default.asp
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

