Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson's disease
- PMID: 18492290
- PMCID: PMC2426681
- DOI: 10.1186/1742-2094-5-19
Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson's disease
Abstract
Background: It has recently become apparent that neuroinflammation may play a significant role in Parkinson's disease (PD). This is also the case in animal paradigms of the disease. The potential neuroprotective action of the glucagon-like peptide 1 receptor (GLP-1R) agonist exendin-4 (EX-4), which is protective against cytokine mediated apoptosis and may stimulate neurogenesis, was investigated In paradigms of PD.
Methods: Two rodent 'models' of PD, 6-hydroxydopamine (6-OHDA) and lipopolysaccaride (LPS), were used to test the effects of EX-4. Rats were then investigated in vivo and ex vivo with a wide range of behavioural, neurochemical and histological tests to measure integrity of the nigrostriatal system.
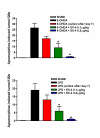
Results: EX-4 (0.1 and 0.5 mug/kg) was given seven days after intracerebral toxin injection. Seven days later circling behaviour was measured following apomorphine challenge. Circling was significantly lower in rats given EX-4 at both doses compared to animals given 6-OHDA/LPS and vehicle. Consistent with these observations, striatal tissue DA concentrations were markedly higher in 6-OHDA/LPS + EX-4 treated rats versus 6-OHDA/LPS + vehicle groups, whilst assay of L-DOPA production by tyrosine hydroxylase was greatly reduced in the striata of 6-OHDA/LPS + vehicle rats, but this was not the case in rats co-administered EX-4. Furthermore nigral TH staining recorded in 6-OHDA/LPS + vehicle treated animals was markedly lower than in sham-operated or EX-4 treated rats. Finally, EX-4 clearly reversed the loss of extracellular DA in the striata of toxin lesioned freely moving rats.
Conclusion: The apparent ability of EX-4 to arrest progression of, or even reverse nigral lesions once established, suggests that pharmacological manipulation of the GLP-1 receptor system could have substantial therapeutic utility in PD. Critically, in contrast to other peptide agents that have been demonstrated to possess neuroprotective properties in pre-clinical models of PD, EX-4 is in current clinical use in the management of type-II diabetes and freely crosses the blood brain barrier; hence, assessment of the clinical efficacy of EX-4 in patients with PD could be pursued without delay.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

